Recording and Editing Raw EEG and Artifact Detection
Client education is the foundation of neurofeedback training (NFT). Professionals can explain core concepts, provide a road map of the NFT process, clarify their respective roles in the training process, and summarize clinic policies. The initial session provides an opportunity to address misconceptions about how NFT works and what the equipment does. Written informed consent is a contract that codifies the terms of your relationship with your client.
Applicants must develop competence in EEG equipment setup and operation. Demonstrations and hands-on training in didactic training programs provide many attendees' first introduction to instrumentation and software. The mentorship relationship builds on this foundation as applicants practice their growing skills on themselves and family members/friends.
EEG equipment literacy requires that applicants understand how to measure the scalp, identify International 10-20 System sites, and attach electrodes. They must understand what a normal raw EEG looks like and gain experience in creating and controlling artifacts so that EEG measurements and brain maps based on them are valid. Finally, applicants must learn to recognize abnormal EEG waveforms and distinguish them from benign activity.

International QEEG Certification Board Blueprint Coverage
This unit covers I. Recording and Editing Raw EEG and Artifact Detection (2 hours).

This unit reviews Client Preparation, Basic Set-up and Operation of EEG Equipment, 10-20 System Electrode Placements, Site Preparation, Elimination of Artifact from EEG Recording, Descriptive Terms, Generally Benign EEG Activity, and Recognizing and Correcting Signals of Noncerebral Origin.
Client Preparation
Essential skills include an overview of client orientation. This includes concepts, major stages, client role and responsibilities, equipment basics, and written informed consent for treatment/training.
Concepts
Explain neurofeedback, self-regulation, and operant conditioning of brainwave activity in the language your client will understand. Adjust the level of explanation to your client's education and experience. Educational handouts and videos like ISNR's Neurofeedback Overview can be invaluable. Hammond's (2011) What is neurofeedback: An update provides a helpful overview for your professional colleagues and the public.Neurofeedback
Anticipate and proactively address common misunderstandings about neurofeedback. The equipment does not run current through your brain, does not change your brain, read your emotions and thoughts, or assess your intelligence, personality, or psychological health.Emphasize that neurofeedback training (NFT) is based on neuroscience. Show pictures of a neuron and the human brain, and explain them in layperson's terms. Then, introduce the concept of neuroplasticity:
Historically the brain has been seen as hard-wired, with each area having its own function. If that area was injured, its function was lost. Today the concept of neuroplasticity has replaced the hard-wired model.
Neuroplasticity refers to changes in neural pathways and synapses which are due to changes in behavior, environment, and neural processes, as well as changes resulting from bodily injury. Neuroplasticity occurs on a variety of levels ranging from cellular changes to large-scale changes involved in cortical remapping. The role of neuroplasticity is widely recognized in healthy development, learning, memory, and recovery from brain damage. (AAPB, 2019)
Present the ISNR Board of Directors' definition of biofeedback (2010):
Biofeedback is a process that enables an individual to learn how to change physiological activity for the purposes of improving health and performance. Precise instruments measure physiological activity such as brainwaves, heart function, breathing, muscle activity, and skin temperature. These instruments rapidly and accurately “feed back” information to the user. The presentation of this information — often in conjunction with changes in thinking, emotions, and behavior — supports desired physiological changes. Over time, these changes can endure without continued use of an instrument.
Emphasize that neurofeedback is a drug-free, surgery-free behavioral intervention.
The goal of biofeedback is to increase your body’s ability to regulate itself. Self-regulation is the ability of your nervous system to respond adaptively to changes in your environment, both internal and external. (Khazan, 2019)
Self-Regulation
Explain that neurofeedback is a mirror that provides immediate information about specific brain activity to treat disorders or improve performance. NFT helps clients change brain patterns to achieve desired cognitive, emotional, and physical changes. While clients are not consciously aware of the neuronal changes, they often learn how successful self-regulation feels and can produce positive states in everyday settings.Operant Conditioning
Explain that NFT utilizes operant conditioning to teach neuronal self-regulation. AAPB's (2019) description might be helpful:Operant conditioning is a method of learning that uses rewards and punishments. The likelihood of a specific behavior is increased through positive or negative reinforcement each time the behavior is exhibited so that the client comes to associate the pleasure of the reinforcement with the behavior.
Over time, the person learns to self-regulate their physiology without the need for a computerized biofeedback device, can perform the behavior outside the clinic, and can create clinical improvement associated with self-regulation.
Major Stages in Neurofeedback Training
Describe intake and assessment, what NFT sessions will be like, scheduling, expected number of sessions, cost and insurance coverage, home practice, and how you will continuously monitor their progress.Client Role and Responsibilities
This stage is critical to NFT training success and client satisfaction. Emphasize that NFT resembles personal training for peak performance. Emphasize that clients must be active participants in the learning process. NFT teaches self-regulation skills; watching displays does not passively change the brain.Describe the exercises they will practice outside of the clinic and why practice is important. Set expectations for practice time, frequency, charting, and accountability. The client's responsibilities should be spelled out in the informed consent agreement.
Explain your clinic's policies concerning privacy, responsibility for payment, session reminders, rescheduling appointments, missed appointments, arriving late to appointments, and leaving sessions early.
Equipment Basics
During the first session, explain what the equipment does and how it works. This should include:(1) the purpose and steps in skin preparation
(2) the function and safety of sensors (avoid the term electrode)
(3) selection of site placements and steps in sensor attachment
(4) the concepts of amplitude and frequency and z-scores
(5) how to understand feedback displays
(6) the relationship between their activity and changes on the feedback display
(7) what will be recorded during a session and how to interpret screens that monitor progress
Written Informed Consent
Written informed consent is a contract between you and your client. State whether the NFT application is experimental or well-supported by peer-reviewed research. AAPB's Evidence-Based Practice in Biofeedback and Neurofeedback (3rd ed.) objectively assesses NFT efficacy. State your clinic's policies, including privacy, insurance, and responsibility for payment. Spell out your respective responsibilities as clinician/coach and client. Explain that you cannot predict the number of training sessions your client will require since each individual is unique. Instead, you will collaboratively assess client progress and the benefit of additional training sessions.Basic Set-up and Operation of EEG Equipment
Critical competencies in qEEG equipment setup and operation include:
(1) measure the scalp and locate each of the International 10-20 system placement sites
(2) correctly prepare the scalp and ears, and attach electrodes to assessment sites or attach an electrode cap for a full-cap qEEG
(3) apply conductive gel to the sensor
(4) make correct hardware connections to the computer
(5) make correct electrode connections to the EEG amplifier
(6) perform all steps required for multi-channel or qEEG recording: checking impedance, performing a tracking test, detecting and removing artifact, and obtaining eyes-open and eyes-closed data
(7) perform a continuity test to ensure electrode integrity
(8) identify and remove (or control) sources of common artifacts that contaminate the raw EEG signal
(9) troubleshoot common equipment failures following the manufacturer's recommendation
(10) demonstrate a basic understanding of multi-channel and qEEG assessment reports as well as components of qEEG databases (absolute power, relative power, phase, coherence, comodulation, and z-score comparisons)
(11) show how to start the software, set NFT protocol parameters, and run basic feedback functions
(12) demonstrate how to set initial training thresholds and adjust as needed
(13) describe how to mitigate the transmission of infection, including scalp measurement, skin preparation, electrode attachment, application of gel, and cleaning of cables, sensors, caps, and exposed surfaces
Proper Electrode Attachment and Location of 10-20 Sites

Review of 10-20 Site Locations
The International 10-20 system is a standardized procedure for electrode placement on 19 scalp and reference and ground sites. Electrodes measure electrical activity from a surrounding area the size of a quarter. The site recorded may be distant from the EEG generator due to neural pathways.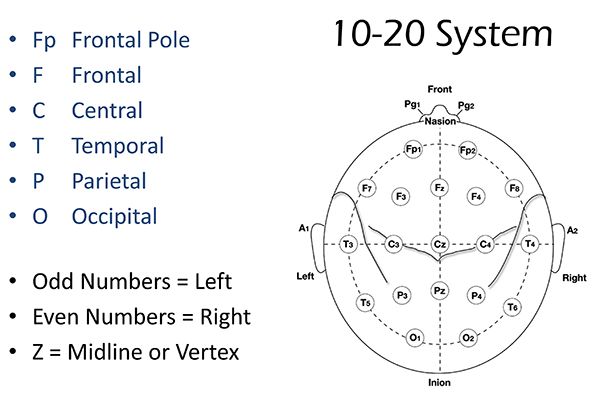
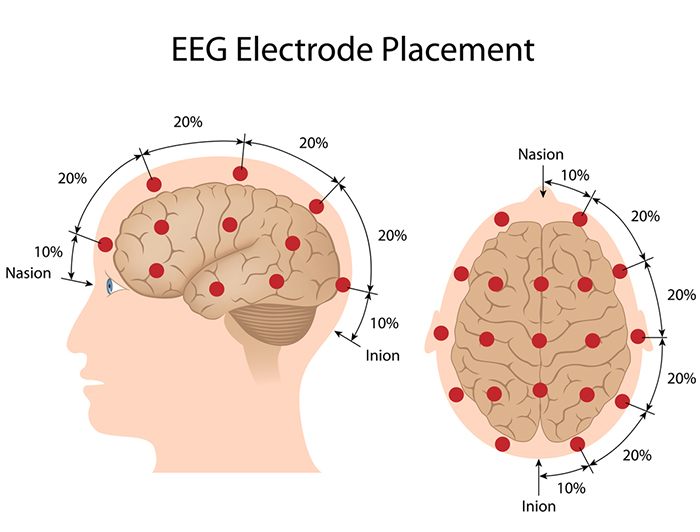
The International 10-20 system calculates the distance from the nasion to the inion and from the left preauricular notch to the right preauricular notch. The 19 active electrode positions are found taking either 10% or 20% of these distances. Check out the YouTube video The International 10-20 System. Four crucial landmarks are the nasion, inion, preauricular points, and vertex. Graphic © Alila Medical Media/Shutterstock.com.
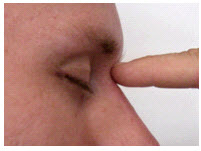
The nasion is the depression at the bridge of the nose.
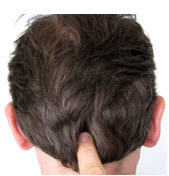
The inion is the bony prominence on the back of the skull in the middle of the inion ridge.
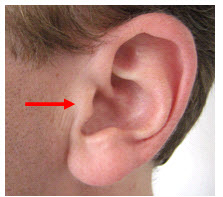
The left and right preauricular points are slight depressions located in front of the ears and above the earlobe. The flap at the opening of the ear is called the tragus.
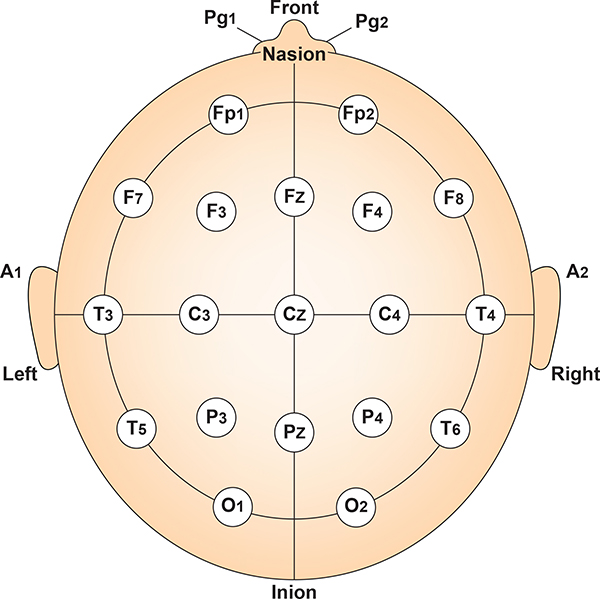
The vertex (Cz) intersects imaginary lines drawn from the nasion to inion and between the two preauricular points. Cz is 50% of the total distance between the nasion and inion and 50% between the two preauricular points. Minaanandag adapted the diagram below from Fisch (1999).
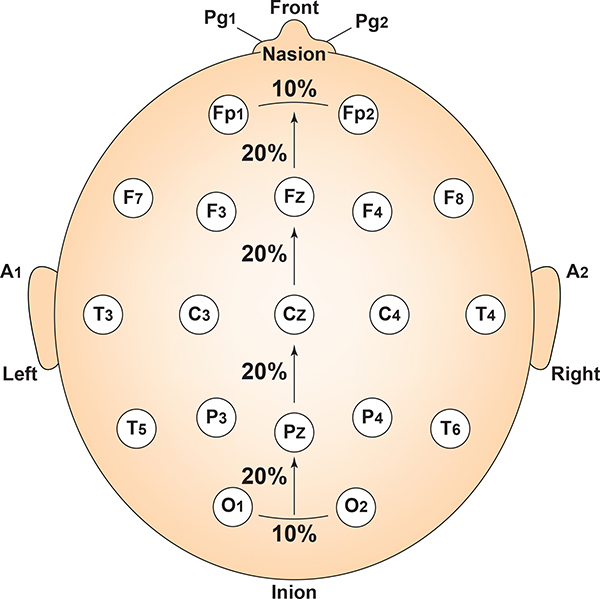
The 10-20 system received its name because electrode sites are separated by 10% or 20% of the distance between two corresponding anatomical landmarks. In the graphic below adapted from Fisch (1999) by minaanandag, each midline site is 10% or 20% of the distance from the nasion to the inion.
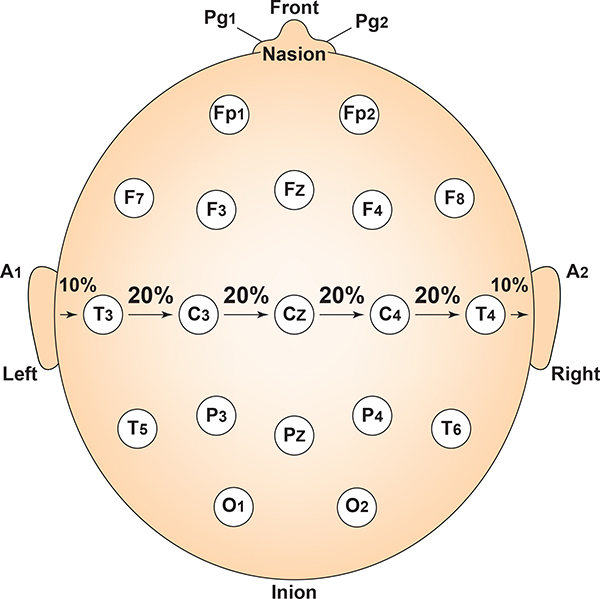
Each horizontal axis site is 10% or 20% of the distance from the two preauricular points. Graphic adapted from Fisch (1999) by minaanandag.
Each circumferential site is 10% of the total circumference, excluding Fpz or Oz. Graphic adapted from Fisch (1999) by minaanandag.
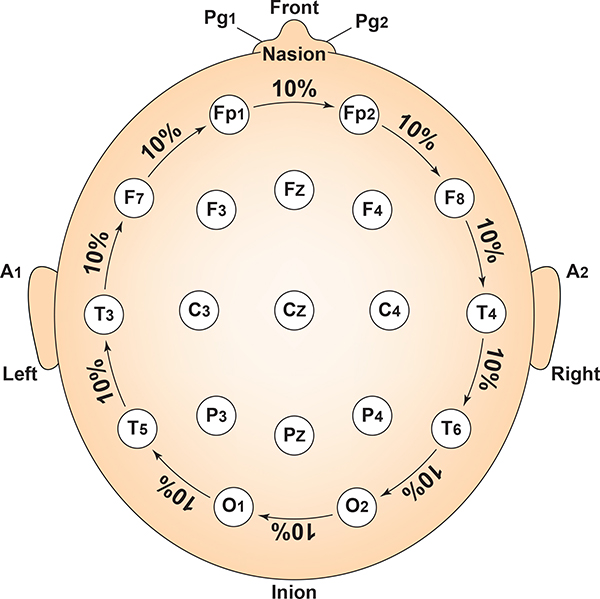
Intermediate sites are halfway between sets of adjacent sites. Graphic adapted from Fisch (1999) by minaanandag.
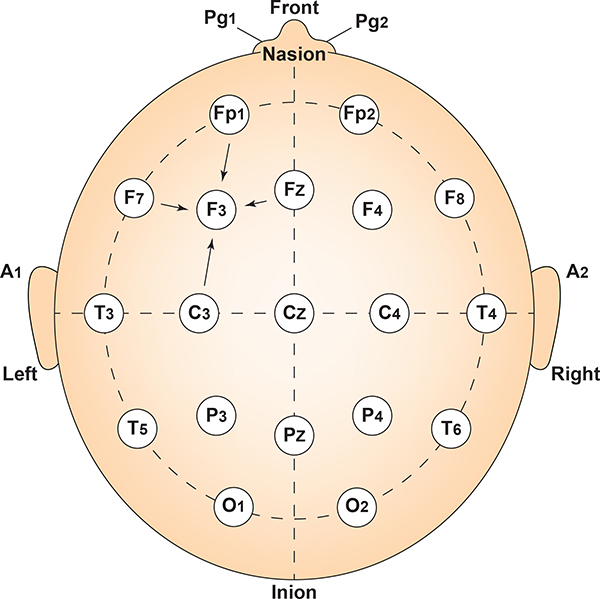
The graphic by Dailey (2013) shows the correspondence between 10-20 sites and Brodmann areas.
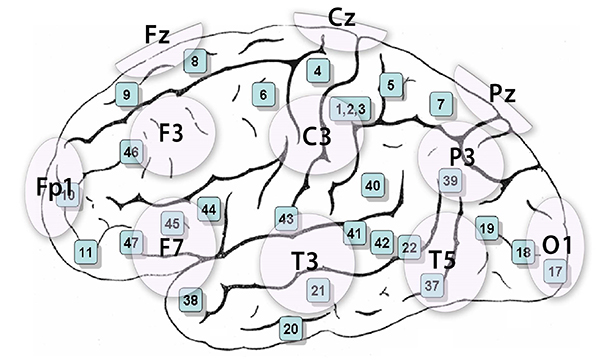
The 10-20 system assigns recording electrodes a letter and subscript. The letters represent the underlying region and include Fp (frontopolar or prefrontal), F (frontal), C (central), P (parietal), O (occipital), and A (auricular). A subscript of z represents a midline (central axis from nasion to inion) placement.
Numerical subscripts range from 1-8 and increase with distance from the midline. The 10-20 system assigns odd-numbered recording electrodes on the left and even-numbered electrodes on the right side of the head. Two reference electrodes are usually placed on the earlobe. John Balven adapted the diagram below from Fisch (1999).
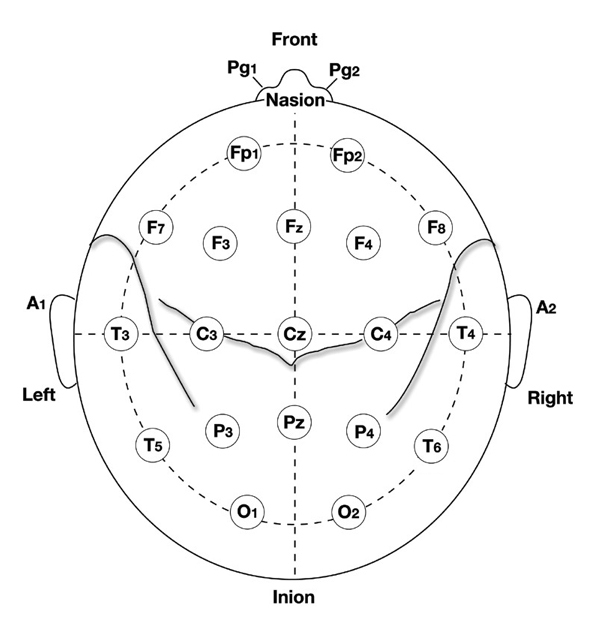
10-20 System Electrode Placements
Short Cuts for Electrode Placement
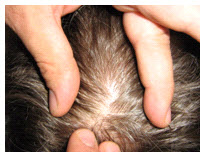
Most sites do not require all the steps outlined below. Calculate Cz by marking 50% of the nasion-inion distance and 50% of the left-right preauricular distance. You can obtain Fz, Pz, C3, C4, T3, and T4 from these two measurements. The Biofeedback Foundation of Europe generously provided the site location, preparation instructions, and graphics.Step-By-Step Guide for Electrode Placement
Consistently mark perpendicularly on the same side of the measuring tape.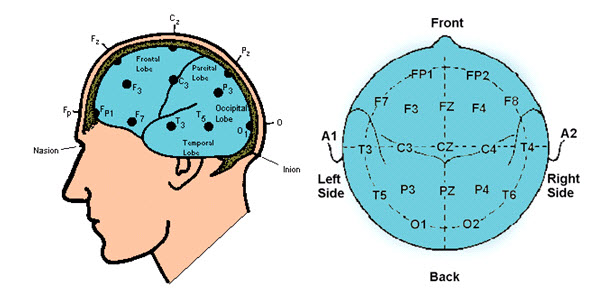
Nasion to Inion – Anterior to Posterior
- Measure from nasion to inion. Note the total and keep the tape measure on this line.
- Mark 50% (or halfway). This is Cz.
- Mark 20% forward from Cz. This is Fz.
- Mark 20% forward from Fz. This is FPz. This should be 10% up from the nasion.
- Mark 20% back from Cz. This is Pz.
- Mark 20% back from Pz. This is Oz. This should be 10% up from the inion.
-
Measure from pre-auricular notch to pre-auricular notch. Find the tragus (the flap at the opening of the ear). Move forward to the indention between the skull and jaw. Place the end of the tape measure at this notch and pass it over Cz and to the pre-auricular notch on the opposite ear. Record this measurement.
- Mark 50% (or halfway). This should intersect with Cz and form a “+.”
- From Cz, mark 20% on each side toward the tragus. These will be C3 and C4.
- From C3 and C4, mark 20% toward the tragus. These will be T3 and T4. These sites will be 10 percent up from
the pre-auricular notch, directly above the ear.
- Place the tape measure on FPz, T3, Oz, T4, and back to FPz. Record the total circumference.
- From FPz, mark 5% on either side. These will be FP1 and FP2.
- From Oz, mark 5% on either side. These will be O1 and O2.
- Mark at 10% increments from FP1 and FP2. These will be F7 and F8.
- Mark at 10% increments from O1 and O2. These will be T5 and T6.
- Measure from Fz to F7: 50% between these points is F3.
- Measure from Fz to F8: 50% between these points is F4.
- Measure from Pz to T5: 50% between these points is P3.
- Measure from Pz to T6: 50% between these points is P4.
Site Preparation
The COVID-19 pandemic has changed site preparation. Now, both clinician and client should wear masks. The clinician should also wear a face shield and gloves. See the unit on Aseptic Techniques for a more comprehensive review of infection mitigation.
Once a site is identified and marked, site preparation and sensor placement when using individual sensors follow these steps:
- Instruct clients to wash their hair and not use any conditioner or hair-styling products. A recent haircut is helpful (particularly for children), and hair must be brushed or combed.
- Prepare the scalp by cleaning with alcohol. Let the alcohol dry before applying the electrodes.
- The following is a typical recommendation when using older amplifiers. Modern amplifiers with high input impedance do not require this step. Additionally, the following step is controversial because the old standard of achieving skin-electrode impedance below 5 Kohms has been challenged as unnecessary and risks infection transmission (Ferree et al., 2001; Kappenman & Luck, 2010).
The authors of this work do not recommend this step, especially during the COVID-19 pandemic. Still, we included it because it remains an accepted standard in the field of electroencephalography: Slightly abrade the skin with a blunted needle that you must discard after use to remove dead skin, dirt, and oil that can weaken the EEG signal. - Ask your client to remove jewelry. If your client has pierced ears, do not place the electrode over the hole.
- Scoop up a small quantity of NuPrep™
skin preparation gel or a similar product on a cotton swab or tissue.

- Be careful to avoid contaminating the gel tube by avoiding direct contact with the Q-tips ®.

- With the thumb and index finger of one hand, separate the hair around the electrode site that was previously
found and marked.
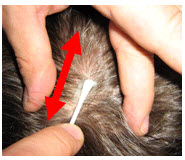
- Run the gel in the direction of the natural line formed along the scalp by the split hair. Some light force must be used, enough to redden the scalp slightly (again, not recommended but an accepted standard), but not enough to break the skin.
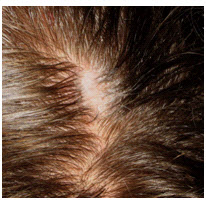
- Wipe away the excess prepping gel with a dry, lint-free cloth. Take care to keep the hair parted and keep
track of the site after wiping clean.
- Use a popsicle or craft stick to cover the electrode with a 1/4-inch layer of Ten20™ conductive paste. Gently press the electrode onto the skin surface until the paste flattens out under the electrode.

- Start by securing the electrode cables to the patient and ensure strain relief.
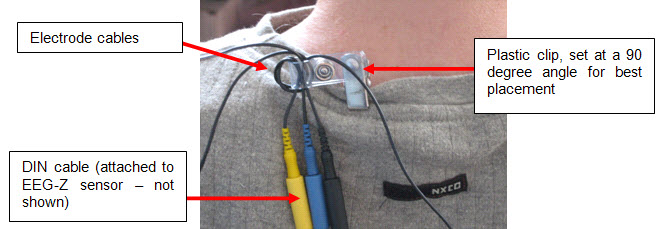
- Fill the electrode cup with Ten20™
conductive paste so that no air bubbles exist in the cup.
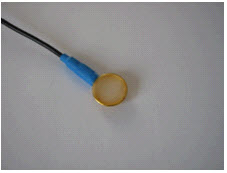
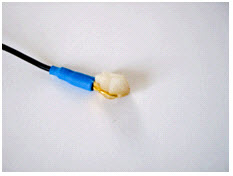
- Add more Ten20™
conductive paste onto the cup electrode, just enough to form a ball on the cup, not so much that it spills
over the edge. The ideal amount of paste is shown.
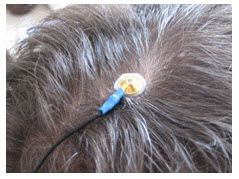
- Place the cup face down on the landing pad previously prepared. Gently push the electrode down to fix it to
the scalp. A little bit of paste should run out along the edge of the cup to form a thin ring around it. Place
the electrode so that the direction of the cable does not place undue stress on the cup (so that it gets
pulled, lifted, or twisted off). The cable should hang naturally and towards the plastic clip (as shown).
Leave enough slack in the cable to allow for comfortable head movement.
- Repeat the above preparation and placement steps on the earlobes. Do not put too much paste on the ear clip
electrode but ensure that the gold disc is completely covered.
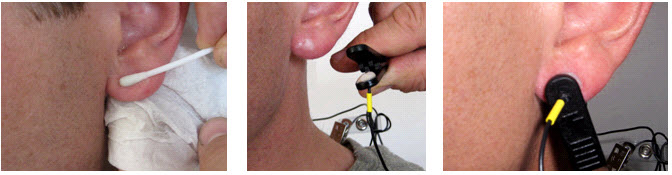
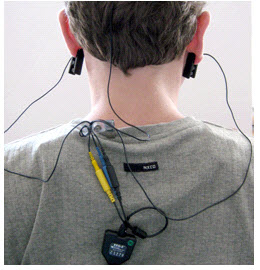
- Leave enough slack in the cable to allow the patient to turn their head easily, but not too much that it can
get caught. Think about the angle of the cable from the ear clip to the neck clip so that no extra tension is
placed on the ear clip. Note the position of the clip, the direction of the cables, and the slack left to
provide mobility. The final configuration should look like the photograph below when viewed from behind.
This movie is a 19-channel BioTrace+ /NeXus-32 display of EEG recording © John S. Anderson.
Elimination of Artifact from EEG Recording
BCIA's Neurofeedback Essential Skills List requires that applicants identify and remove artifact sources appearing in EEG recordings. Review the Signal Acquisition unit to recognize normal EEG patterns and identify and correct noncerebral origin signals like bridging artifacts. Once you understand the mechanics and appearance of common artifacts, Peper et al. (2008) recommend that you intentionally reproduce them to recognize and prevent them.
Brain map validity depends on the integrity of the raw EEG. You will need 45 seconds of reasonably clean data to construct a valid brain map from raw EEG recordings 3-10 minutes in length. However, the ideal amount of clean data is approximately 2 minutes for adequate database comparison. Realize that longer recordings risk increased drowsiness artifacts and sleep. Therefore, it is helpful to speak to the client to help them maintain alertness occasionally. Simply saying how much time is left every 1-2 minutes is usually enough to accomplish this.
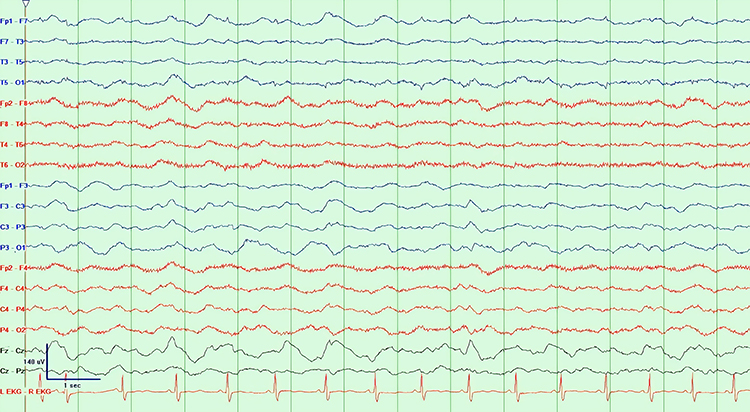
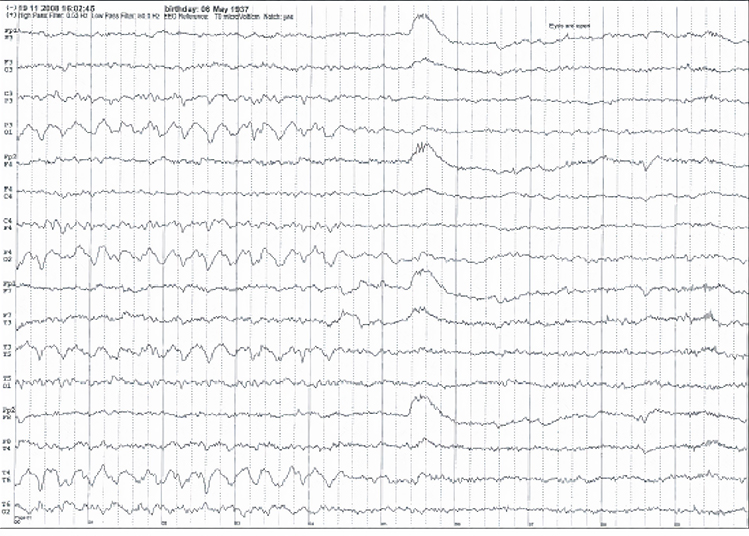
Stage 1 sleep is a subtle drowsy state that clients often do not recognize. This state change is seen as a decrease in alpha and an increase in theta amplitudes. There will be slow eye-rolling movements and decreased EMG and beta. Stage 1 sleep graphic © John S. Anderson. Note the increased theta amplitudes in the spectral displays for channels 1 and 2.
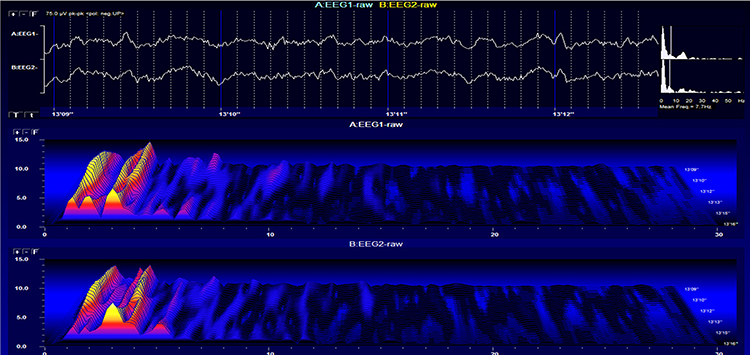
The less frequent the artifact, the shorter the required recording period. Disable low-pass and high-pass filters before editing to better visualize electro-ocular and SEMG artifacts.
Worst case, as with a hyperactive child, none of the EEG channels may contain usable data, and you will need to repeat the assessment. Where artifact only contaminates a few channels, you may base assessment on the clean channels (Demos, 2019).
Strategies to Reduce Artifact
Demos (2019) recommends several precautions to reduce artifact in raw EEG recordings:- Demonstrate how to create artifacts for your clients using screen displays while they clench their teeth, move their eyes, blink, swallow, and fidget
- Confirm the cap fits properly
- Use reclining chairs with negligible neck cushioning that can force the head downward to minimize SEMG artifact
- Limit eyelid movement with cotton balls gently touching the closed eyelids, secured by a loose sleep mask, flexible band, or tape in the eyes-closed recording. There should be no pressure against the eyes
- Ensure that impedance values or DC offset values are appropriate for your amplifier (values under 5 Kohms are expected for publishable research, but values of less than 20 Kohms are acceptable for general clinical sessions and do not require excessive skin abrasion)
- Only record qEEG data when the raw waveforms appear clean
The movie is a 19-channel BioTrace+ /NeXus-32 display of EEG processing in NeuroGuide™ © John S. Anderson.
Recognition of Spike/Wave Activity in the Raw EEG
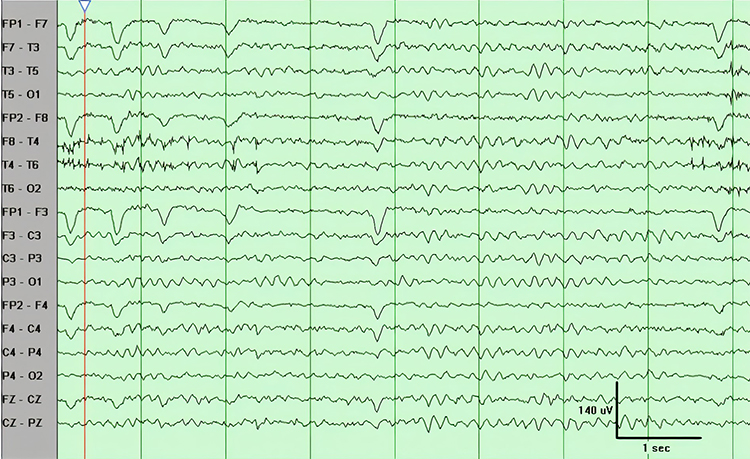
Abnormal EEG patterns include abnormal slow activity, paroxysmal epileptogenic abnormalities, and abnormal periodic paroxysmal patterns.Abnormal Slow Activity
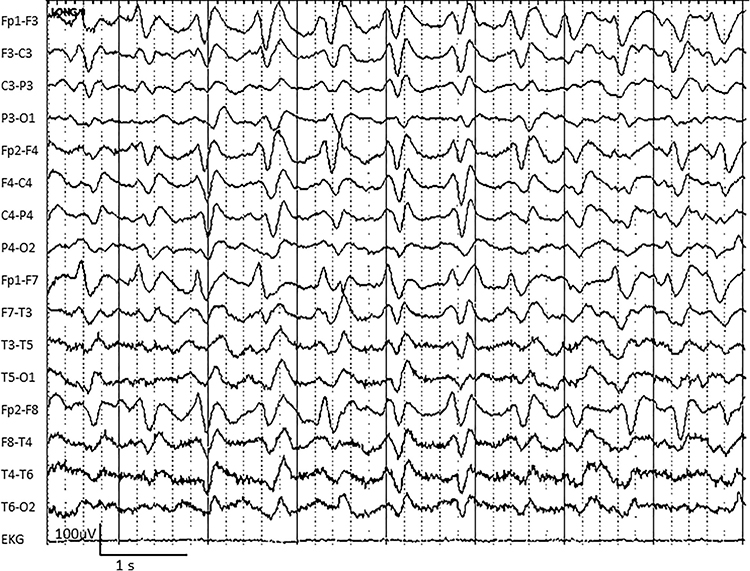
Abnormal slow activity includes generalized intermittent slow activity, focal and lateralized intermittent slow activity, and persistent slow activity.Generalized intermittent slow activity is asynchronous, under 8 Hz, and involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta (Benbadis & Rielo, 2018). Alerting and opening the eyes reduce, whereas hyperventilation challenge and relaxation increase these slow waves (Fisch, 1999). Graphic © Medscape.
Focal and lateralized intermittent slow activity is under 8 Hz and is usually confined to a single or a couple of adjacent electrodes. These bursts have an irregular appearance, are composed of several frequencies, and rarely involve an entire hemisphere (Fisch, 1999). Graphic © eegatlas-online.com.
Persistent slow activity consists of theta and delta waveforms. Distribution may be anterior or widespread in encephalopathies (Richardson & Benbadis, 2019). Graphic © Medscape.
Paroxysmal Epileptogenic Abnormalities
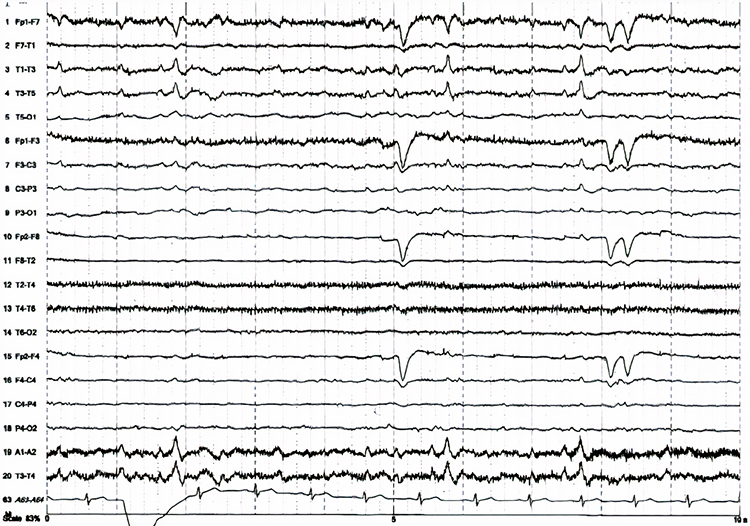
Paroxysmal epileptogenic abnormalities include interictal epileptiform discharges (focal, generalized), ictal, secondary bilateral synchrony, and epileptiform patterns of doubtful significance.Interictal epileptiform discharges typically consist of individual spikes and sharp waves and complexes that contain both waveforms that last less than 2 seconds (Fisch, 1999). Graphic courtesy of Teppei Matsubara.
Ictal epileptiform discharges may consist of prolonged interictal activity. These may include 3-Hz spike-and-wave discharges, slow spike-and-wave discharges, sharp-and-slow-wave discharges, amplitude and frequency fluctuations in rhythms of 10 Hz or higher, and irregular multiple spike-and-wave or spike-and-wave discharges (Fisch, 1999). Graphic © eegatlas-online.com.
Secondary bilateral synchrony (SBS) involves spikes with a single phase reversal around the midline (Jin, 2007). Graphic © Neurology Asia.
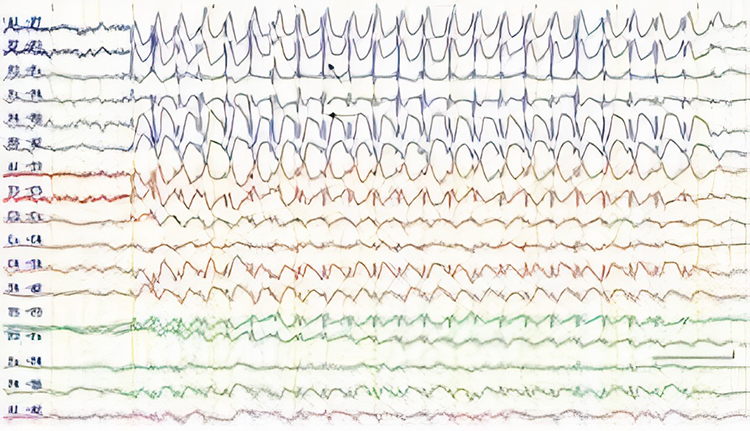
Epileptogenic patterns of doubtful significance are brief and not associated with seizures or neurological disorders. Examples are 6-Hz spike-and-slow-wave, 14- and 16-Hz positive bursts, benign epileptiform transients of sleep (BETS), rhythmical mid-temporal discharge (RMTD), small sharp spikes (SSS), and wicket spikes (Fisch, 1999). Six-Hz graphic © Mayo Foundation for Medical Education.
Abnormal Periodic Paroxysmal Patterns
Abnormal periodic paroxysmal patterns include generalized periodic paroxysmal patterns and lateralized periodic paroxysmal patterns. Generalized periodic paroxysmal patterns involve the same areas of both hemispheres. The waveforms exhibit similar composition, amplitude, and phase in each hemisphere but may slightly vary within a hemisphere (Fisch, 1999). Graphic © Epilepsy & Behavior.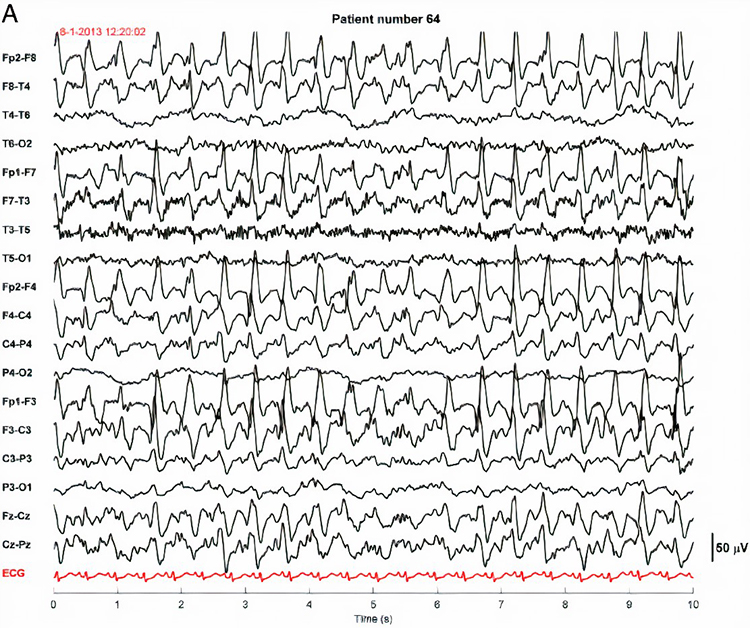
Lateralized periodic paroxysmal patterns differ from generalized periodic paroxysmal patterns in their unilateral distribution. Both patterns share the same waveform morphology (Fisch, 1999). Graphic © Internal Medicine.
Descriptive Terms
Amplitude is classified as low (less than 20 µV), typical (20-50 µV, depending on age), and high (60-200+ µV). Duration is described as very brief (less than 10 s), brief (10-60 s), intermediate duration (1-1.5 min), prolonged (5-60 min), and protracted (greater than 60 min).
Common EEG Acronyms for Rhythmic and Periodic Patterns
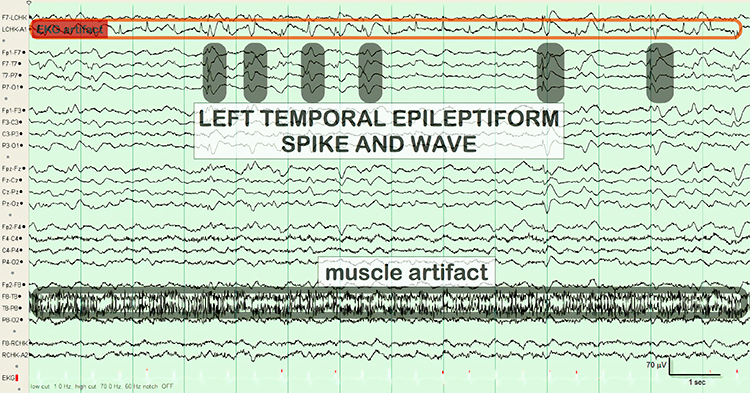
The common EEG acronyms reviewed in this section include LPDs, BIPDs, GPDs, GRDA, IRDA, LRDA, PLEDs, BIPLEDs, FIRDA, GPEDs, Mf, SIRPIDs, and SW.LPDs are lateralized periodic discharges. These unilateral discharges appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz (Johnson & Kaplan, 2017). Graphic © ScienceDirect.
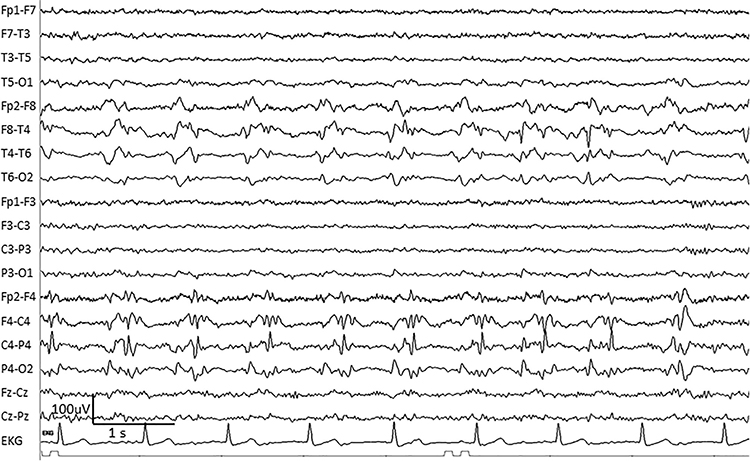
BIPDs are bilateral independent periodic discharges. These asynchronous discharges occur independently in the left and right hemispheres, appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz (Johnson & Kaplan, 2017). Graphic © ScienceDirect.
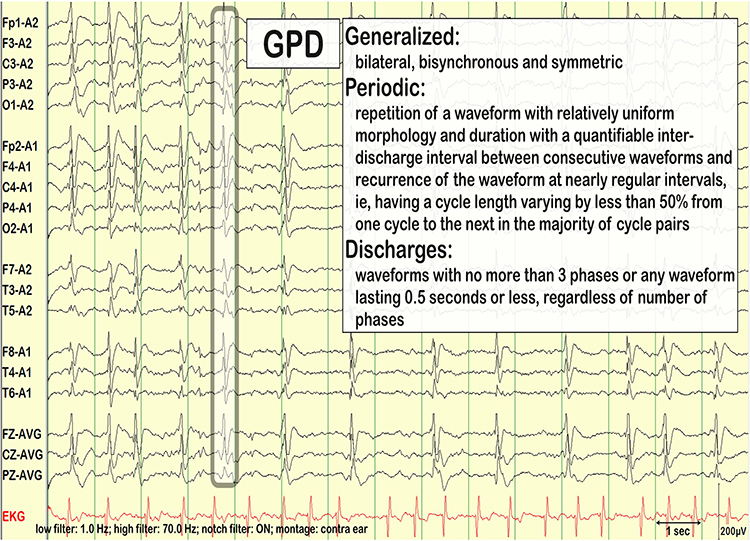
GPDs are generalized periodic discharges. These discharges synchronously occur in both hemispheres, appear as sharp waves or spikes, amplitudes exceed 100 µV, and recur at rates up to 3 Hz (Johnson & Kaplan, 2017). Graphic © ScienceDirect.
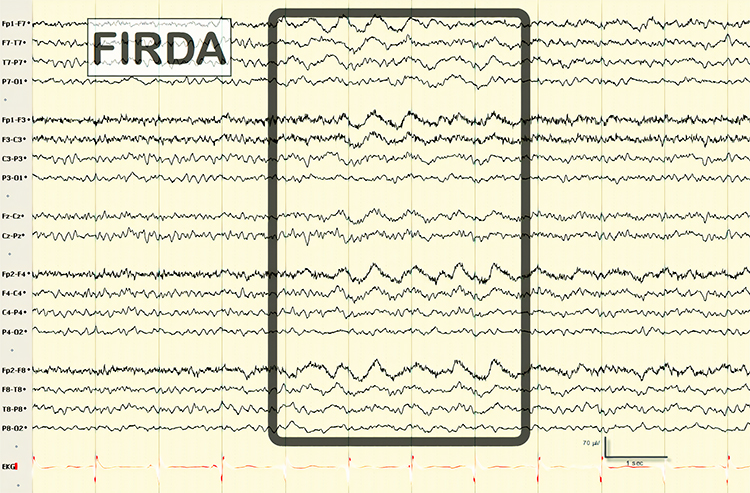
GRDA is generalized rhythmic delta activity. The term frontal intermittent rhythmic delta activity (FIRDA) was used before ACNS standardization in 2012. These bilateral and synchronous discharges exceed 100 µV and recur at rates up to 3 Hz (Johnson & Kaplan, 2017). Graphic © ScienceDirect.
For focal patterns, describe the location with R (right). L (left), A (anterior), and P (posterior).
IRDA is intermittent rhythmic delta activity. These bilateral synchronous discharges recur between 2-2.5 Hz and appear in brief bursts. Graphic © Richardson and Benabis (2019).
LRDA is lateralized rhythmic delta activity. These unilateral discharges recur at rates up to 3 Hz. LRDA runs typically last less than 1 minute and is shorter than LPDs (Johnson & Kaplan, 2017). Graphic © ScienceDirect.
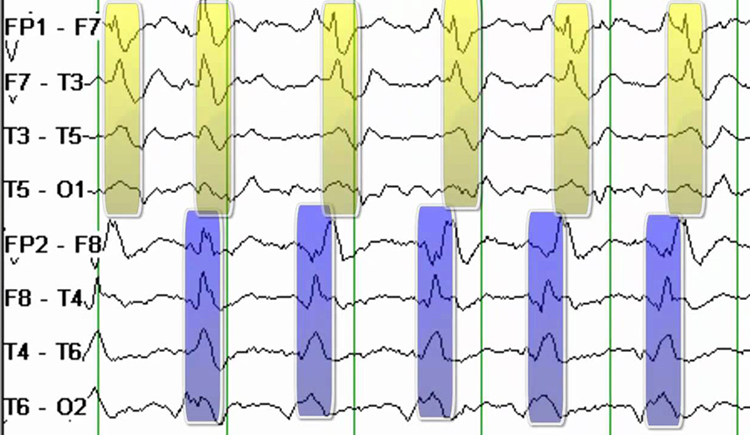
PLEDS are periodic lateralized epileptiform discharges. They are lateralized or focal and exhibit regular periodic, negative spike-and-sharp wave patterns with a 20-1000 ms duration and 50-300 µV amplitude.
BIPLEDS are bilateral independent periodic lateralized epileptiform discharges. They are asynchronous discharges that occur independently in both hemispheres, appear as sharp waves or spikes, 40-100 µV in bipolar montages, and recur at rates from 0.5-1.5 Hz.
FIRDA is frontal intermittent rhythmic delta activity. Structural brain lesions and encephalopathy are independently associated with the occurrence of FIRDA. Asymmetric FIRDA may be associated with an underlying brain lesion.
FIRDA appears more common than previously reported and is associated with various lesions and encephalopathic conditions. However, FIRDA may also occur in otherwise healthy subjects during hyperventilation. FIRDA occurrence should prompt investigations for toxic-metabolic disturbances and structural lesions (particularly if asymmetric) but does not suggest an epileptic risk. Graphic © eegatlas-online.com.

GPEDs (or GPDs) are generalized periodic epileptiform discharges. Graphic © eegatlas-online.com.
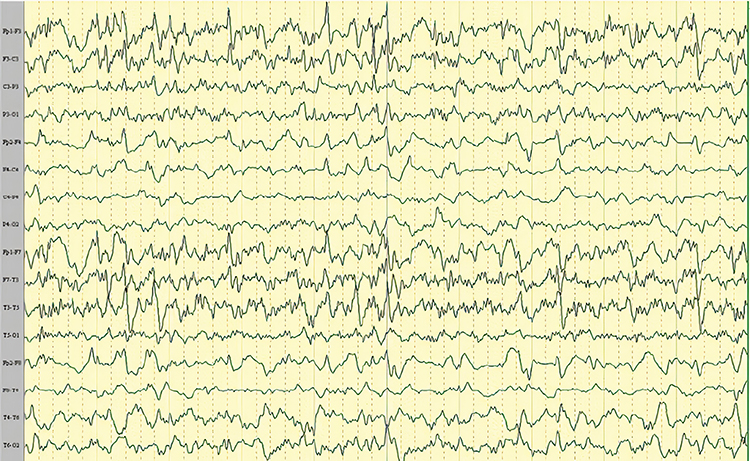
Mf is multifocal. Graphic © Tai-Tong Wong.
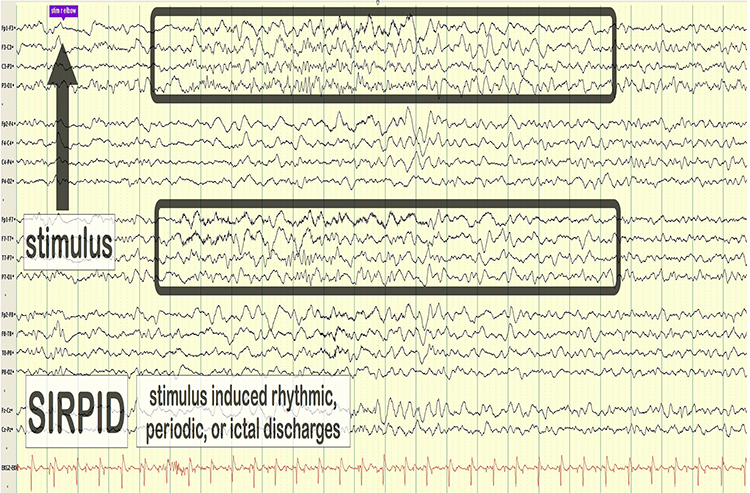
SIRPIDs are stimulus-induced rhythmic, periodic, or ictal discharges. Graphic © eegatlas-online.com.
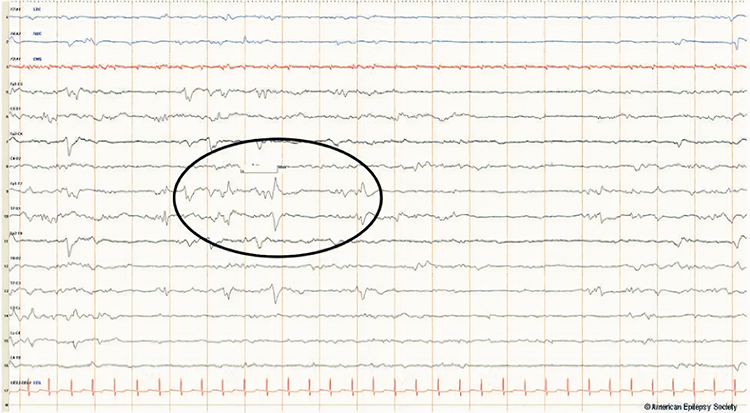
SW is spike-wave or sharp-wave.
Generally Benign EEG Activity
Generally benign EEG activity includes BETS, POSTS, RMTD, SREDA, and wicket waves.
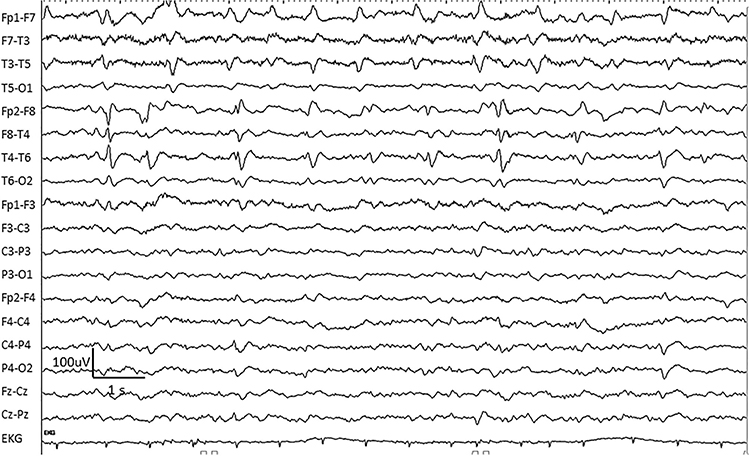
BETS are benign epileptiform transients of sleep. This activity is also called BSSS for benign small sharp spikes or benign sporadic sleep spikes. Sharp waves are seen alone or as a low-amplitude spike and a smaller after-going slow wave. BETS can be monophasic or diphasic and occur during light sleep. There is no disturbance of background activity, and it does not progress. BETS often appears in an ear reference, and when seen elsewhere, it is due to reference contamination. BETS are seen in adults during drowsiness, but it disappears in deeper sleep.
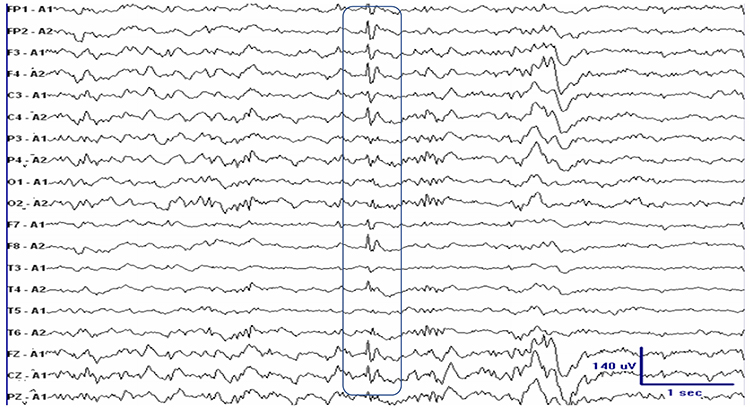
POSTS are positive occipital sharp transients of sleep. They are sharply contoured with a frequency of 4-5 Hz and seen alone or in groups in occipital areas. POSTS are usually bilaterally synchronous but opposite in phase. Graphic © eegatlas-online.com.
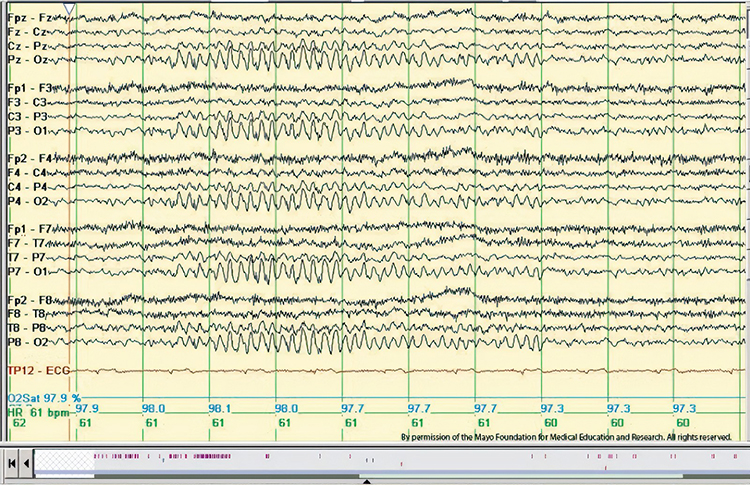
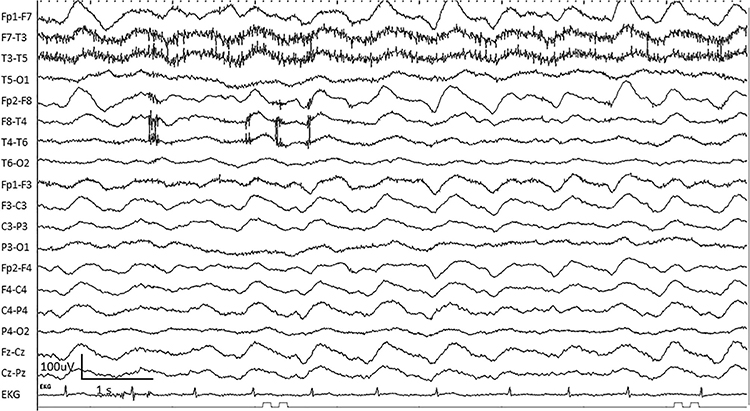
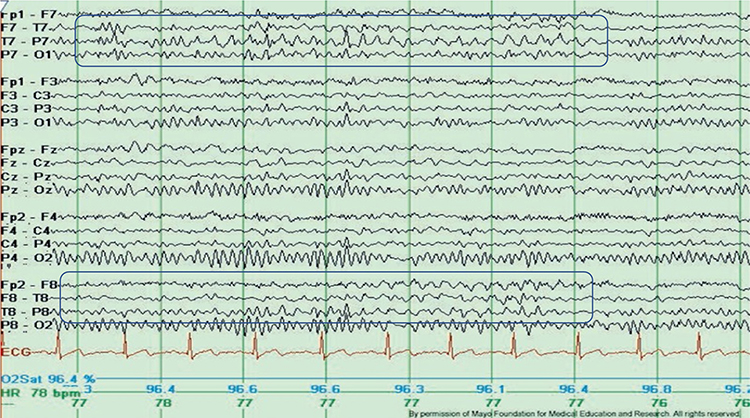
RMTD is the rhythmic temporal theta of drowsiness. Bitemporal left is greater than right in this longitudinal bipolar montage. Noted are notched rhythmic waveforms localized to the temporal regions, some of which are sharply contoured. This rhythm was formerly referred to as the “psychomotor variant,” which can be differentiated from an epileptiform discharge by its relatively monomorphic appearance, lack of clinical accompaniment, and lack of spatiotemporal evolution. Graphic © Mayo Foundation for Medical Education and Research. Figure courtesy of Jeffrey W. Britton, MD.
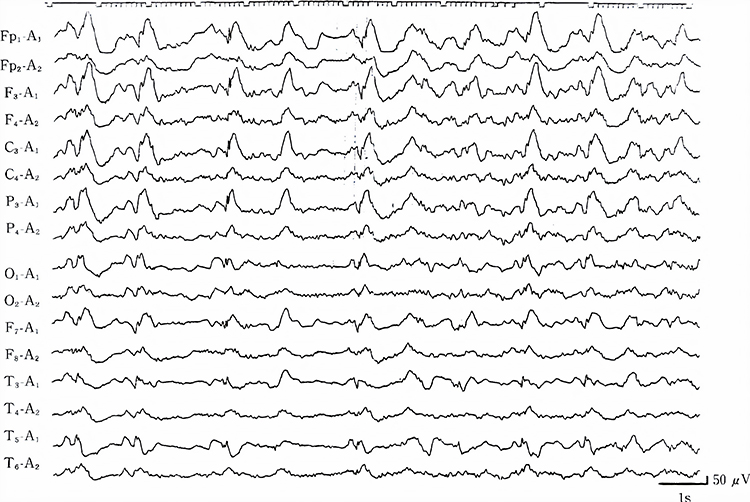
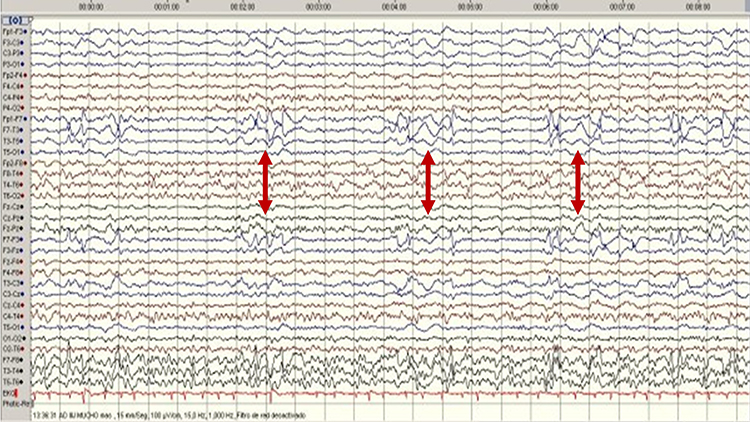
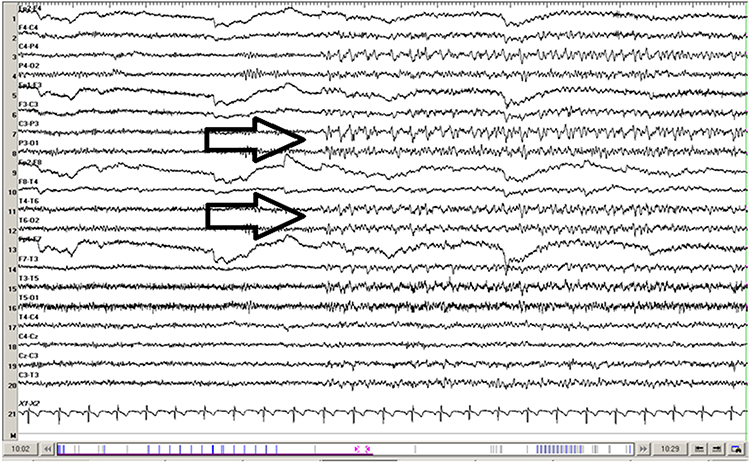
SREDA (or SCREDA) is sub-clinical rhythmic electrographic discharges in adults. In the bilateral synchronous parieto-temporal sharp theta rhythm graphic below, black arrows show the onset of periodic posterior-predominant sharply contoured waveforms. The waveforms become rhythmic and then resolve at the latter portion of the figure.
Wicket waves exhibit an arciform appearance, no after-going slow wave, and no background disruption or disturbance. The wicket waves in the graphic below are seen in the left temporal region with phase-reversal at T7 in seconds 3 and 4 of the tracing using longitudinal bipolar montage. Graphic © Mayo Foundation for Medical Education and Research. Figure courtesy of Jeffrey W. Britton, MD.
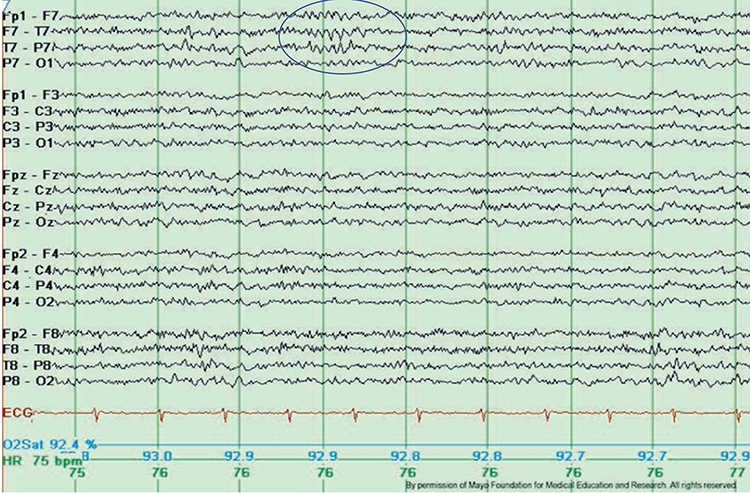
Recognizing and Correcting Signals of Noncerebral Origin
EEG artifacts, consisting of noncerebral electrical activity, can be divided into physiological and exogenous artifacts. Physiological artifacts include electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential. Exogenous artifacts include movement, 60 Hz and field effect, and electrode (impedance, bridging, and electrode pop) artifacts.
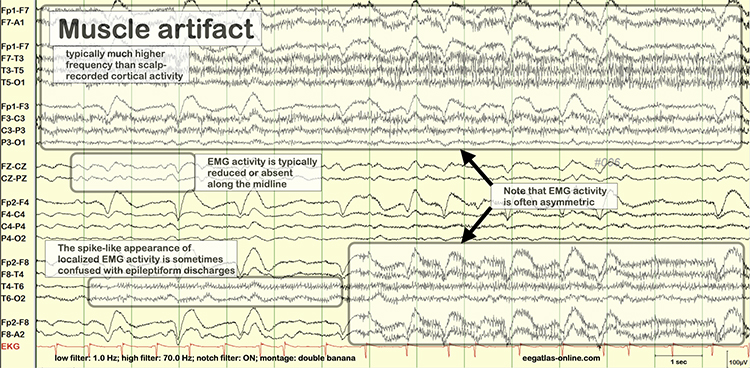
Electromyographic (EMG) Artifact
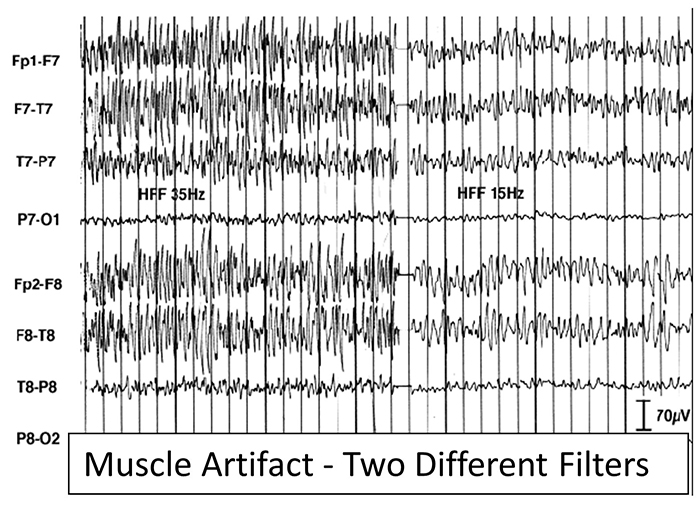
EMG artifact is interference in EEG recording by volume-conducted signals from skeletal muscles. This artifact contains high-frequency activity that resembles a "buzz" of fast activity during a contraction. EMG is seen as fast beta activity in the qEEG. While some frequencies are between 10-70 Hz, most are 70 Hz or higher.The graphic shows how high-frequency filter (HFF) selection can affect contamination by this artifact. A high-frequency filter (low-pass filter) attenuates frequencies above a cutoff frequency. In the examples below, the cutoffs are 35 Hz and 15 Hz.
All the channels on the left side of the tracing show SEMG artifact admitted by a 35-Hz high-frequency filter. The right tracing is free from SEMG artifact since its 15-Hz high-frequency filter attenuates the higher frequencies that contain this artifact.
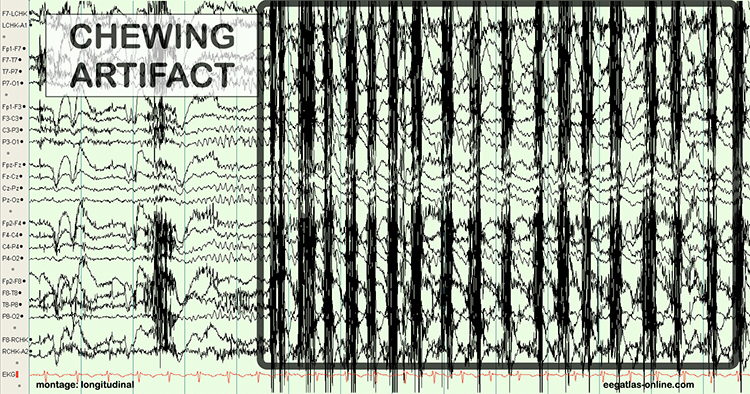
The next graphic shows how gum chewing can generate SEMG artifact by contracting the muscles of mastication. Graphics © eegatlas-online.com.
The frequency spectrum for SEMG artifact ranges from 2-1,000 Hz. While strong muscular contraction can contaminate all frequency bands, including 10 Hz, the beta rhythm (at 70 Hz or higher) is most affected by this artifact. EMG artifact may create the appearance of greater beta activity than is present. Graphic © eegatlas-online.com.
Below is a BioGraph ® Infiniti EMG artifact display. Note how the amplitude of the EEG spectrum increases with each contraction.
Thompson and Thompson (2016) observed that EMG artifact is readily detected because it affects one or two channels, particularly at T3 and T4 at the periphery, and less often at O1, O2, Fp1, and Fp2.
You can identify EMG artifact by visually inspecting the raw signal. The next graphic shows SEMG artifact using a 70-Hz high-frequency filter.
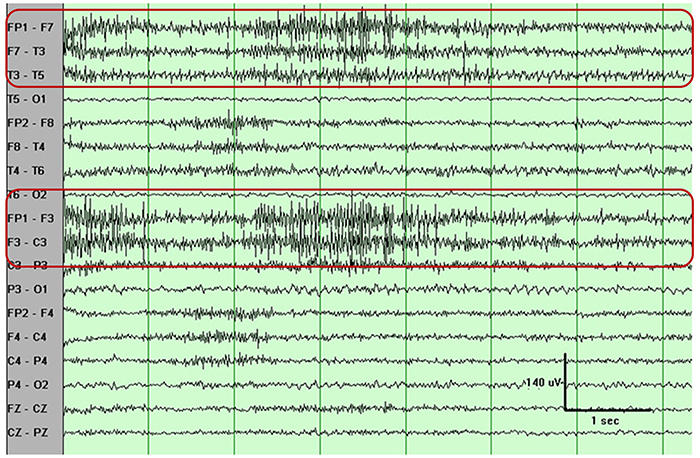
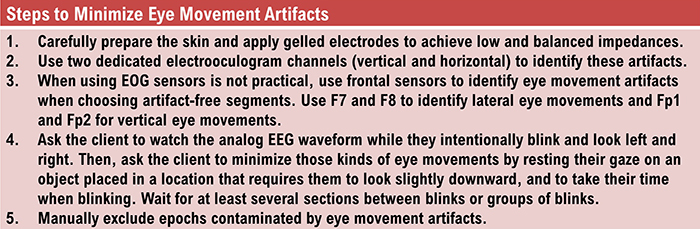
Electro-Ocular Artifact
Electro-ocular artifact contaminates EEG recordings with potentials generated by eye blinks, eye flutter, and other eye movements. For example, anxious patient eyelid flutter may cause deflections at Fp1 and Fp2 (Klass, 2008).This artifact is due to the movement of the eye’s electrical field when the eye rotates and the contraction of the extraocular muscles. The eye creates a dipole that is electropositive at the front and electronegative at the back. Bell’s Phenomenon refers to the upward rotation of the eye when it closes and causes an artifact seen as an apparent increase in EEG.
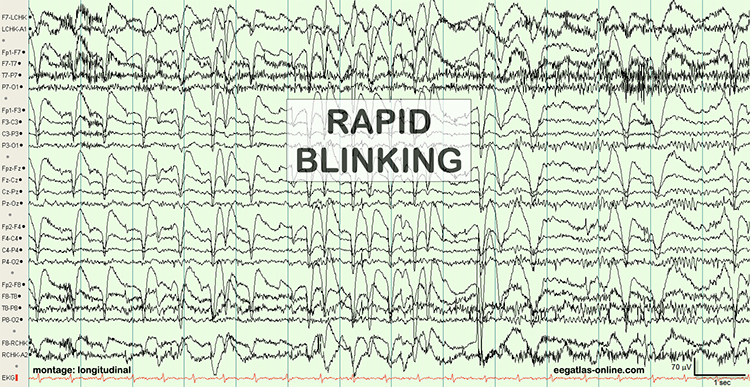
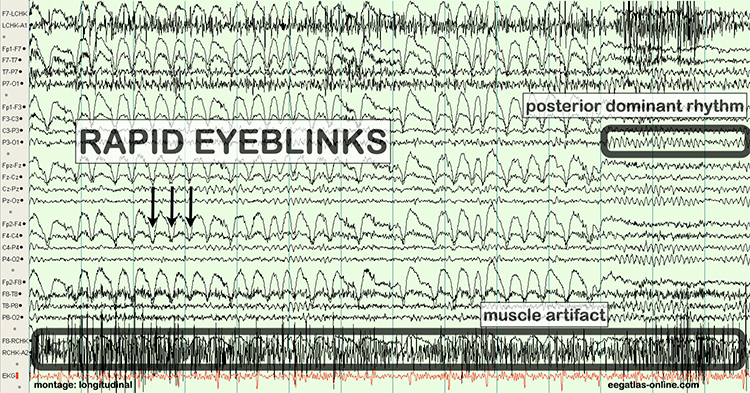
The next two graphics show eye movement artifacts due to rapid blinking. Graphics © eegatlas-online.com.
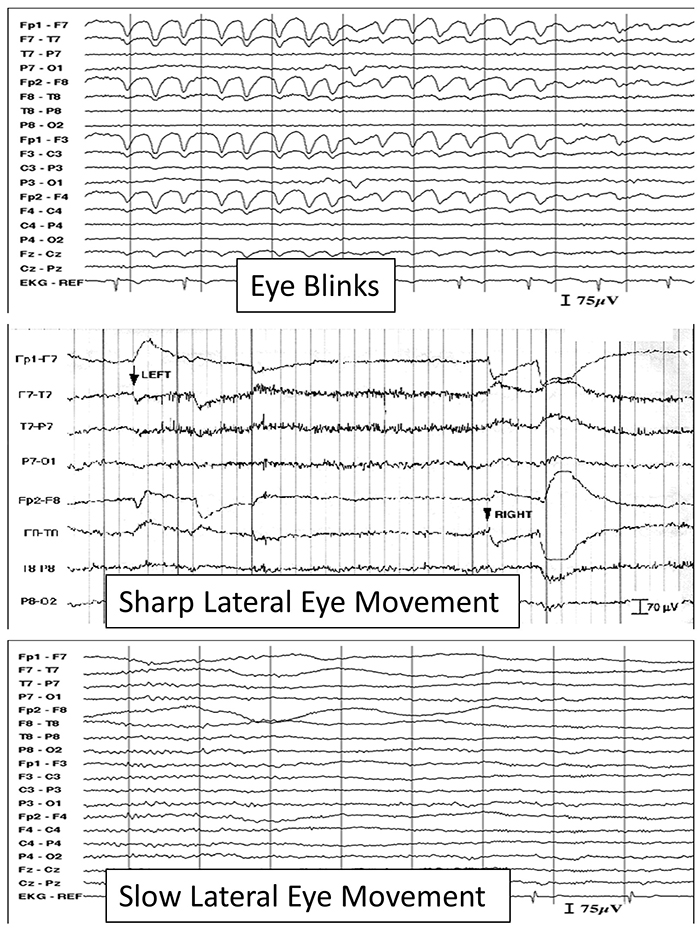
The next graphic shows eye blinks, sharp lateral eye movement, and slow lateral eye movement.
Below is a BioGraph ® Infiniti EEG display of eye movement artifact.
Below is a NeXus display of eye blink and EMG © John S. Anderson.
An upward eye movement will create a positive deflection at Fp1, while a downward eye movement may create a negative deflection. In a longitudinal sequential montage, the artifact is typically seen at frontal sites (Fp1- F3 and Fp2-F4). A left movement may produce a positive deflection at F7 and a negative deflection at F8 (Thompson & Thompson, 2015).
Rapid eye flutter may resemble seizure activity. Graphic © John S. Anderson.
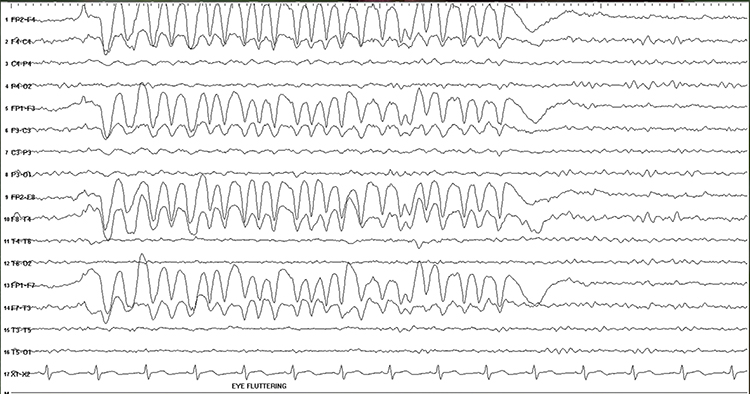
Cardiac and Pulse Artifacts
Cardiac artifact occurs when the ECG signal appears in the EEG. This artifact may be produced when electrode impedance is imbalanced or too high or when an ear electrode contacts the neck. The frequency range for ECG artifact is 0.05-80 Hz, and it contaminates the delta through beta bands. Since multiple electrodes detect this artifact, it can create the appearance of greater coherence than is present. Graphic © eegatlas-online.com.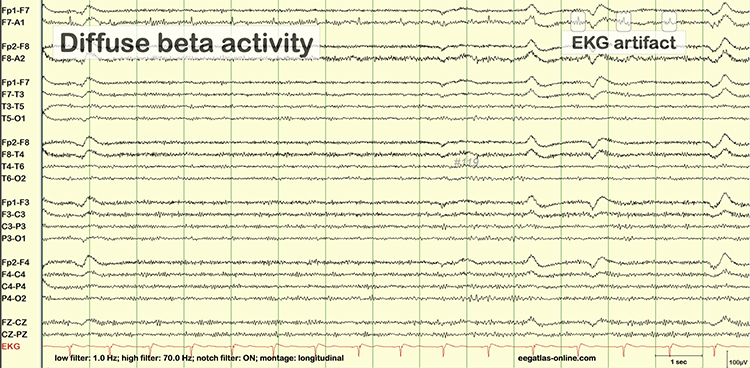
You can detect cardiac artifacts by inspecting chart recorder, data acquisition, or oscilloscope displays of the raw EEG waveform. Cardiac artifact appears as a wave that repeats about once per second (Thompson & Thompson, 2016). Below is a BioGraph ® Infiniti ECG artifact display.
Pulse artifacts are due to the mechanical movement of an electrode in relation to the skin surface due to the pressure wave of each heartbeat. Graphic © eegatlas-online.com.
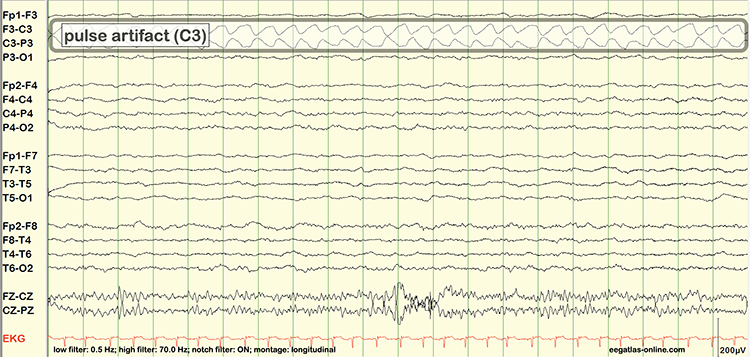
Sweat (Impedance) Artifact
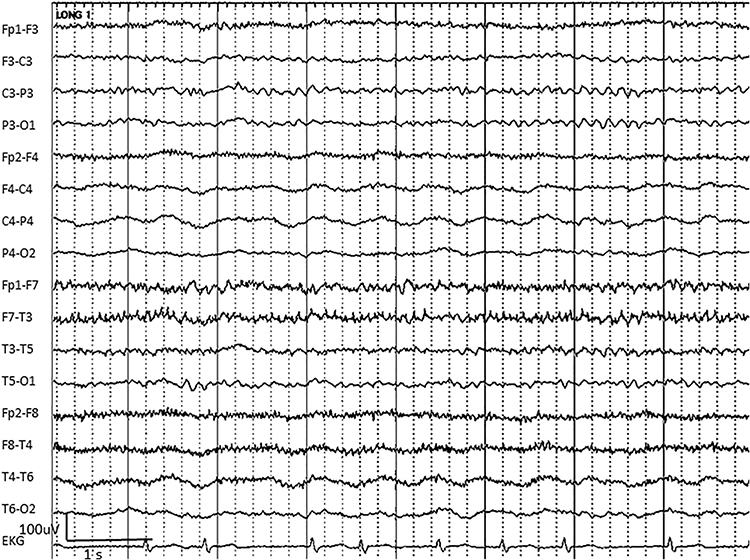
Sweat artifact results from sweat on the skin changing the conductive properties under and near the electrode sites (i.e., bridging artifact). Sweating reduces electrode contact with the scalp and generates large-scale up and down EEG line movements in several frontal channels. This artifact is often elicited by abrupt, unexpected stimuli and usually appears as isolated 1-2 Hz slow waves of 1-2-s duration at frontal and temporal sites (Thompson & Thompson, 2015). Graphic © eegatlas-online.com.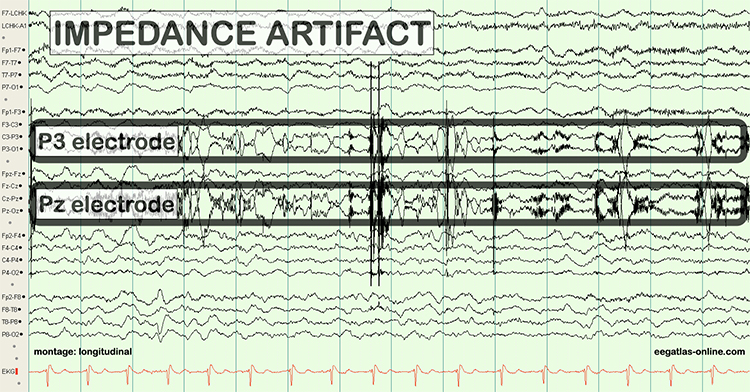

Bridging Artifact
A short circuit produces bridging artifact between adjacent electrodes due to excessive application of electrode paste or a client who is sweating excessively or who arrives with a wet scalp. Bridging artifacts can cause adjacent electrodes to create a short circuit that produces identical referential EEG recordings or a flat line with a bipolar montage. The Fp1-F3 channel's reduced amplitude and frequency illustrate a bridging artifact.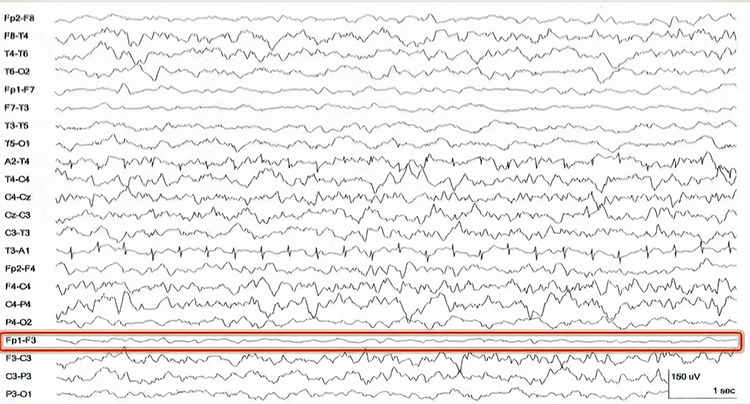
Drowsiness Artifact
Drowsiness artifact is the appearance of stage 1 or stage 2 sleep in the EEG. Stage 1 and stage 2 of sleep are most likely during eyes-closed recording. Sleep may occur during eye-closed awake recording. Graphic © eegatlas-online.com.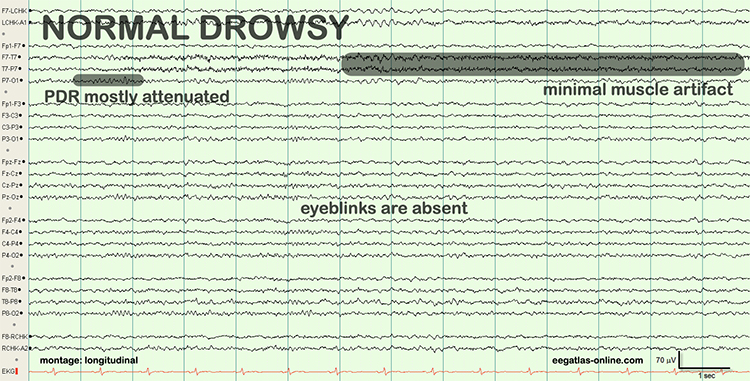
Stage 1 sleep is a subtle drowsy state which clients often do not recognize. Alpha amplitude (especially occipital) may decrease, and theta (especially frontal) may increase. Reductions in EMG and beta amplitude will accompany slow eye-rolling movements. Sleepiness may be accompanied by spike-like transients (vertex or V-waves). The graphic below © John S. Anderson shows increased theta during stage 1 sleep.
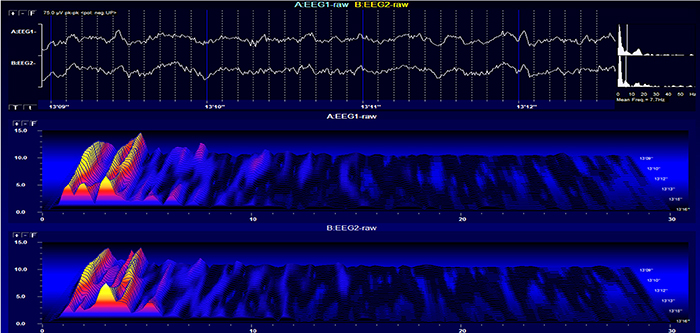
When you detect drowsiness artifact during a training session, suspend recording and instruct your clients to move their hands and legs to increase wakefulness. To avoid this artifact, ask them to retire early and sleep for 9 hours if possible (Thompson & Thompson, 2003).
Evoked Potential
Evoked potential artifact (also called event-related potential artifact) consists of somatosensory, auditory, and visual signal processing-related transients that may contaminate multiple channels of an EEG record. While evoked potentials increase recording variability and reduce its reliability, they minimally affect averaged data (Thompson & Thompson, 2015).The graphic below is courtesy of BPM biosignals' YouTube video EEG: Visually evoked potentials (VEP).
Movement Artifact
Movement artifact is caused by client movement or the movement of electrode wires by other individuals. Most of these artifacts are produced by brief changes in electrode-skin surface connection. Cable movement is called cable sway. The graphic below that illustrates cable sway is courtesy of iMotions.com.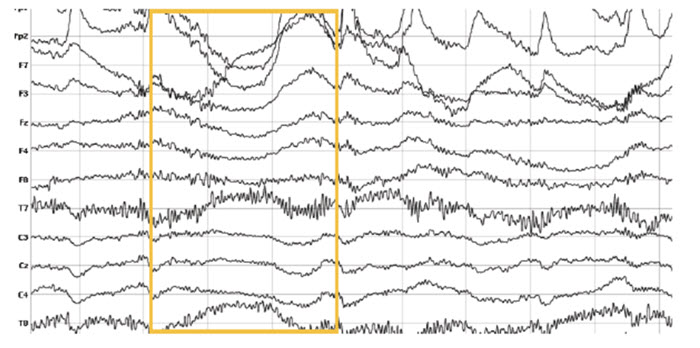
Movement artifacts can produce high-frequency and high-amplitude voltages identical to EEG and EMG signals. While the delta rhythm is most affected by this artifact, it may also contaminate the theta band (Thompson & Thompson, 2016). Graphic © eegatlas-online.com.
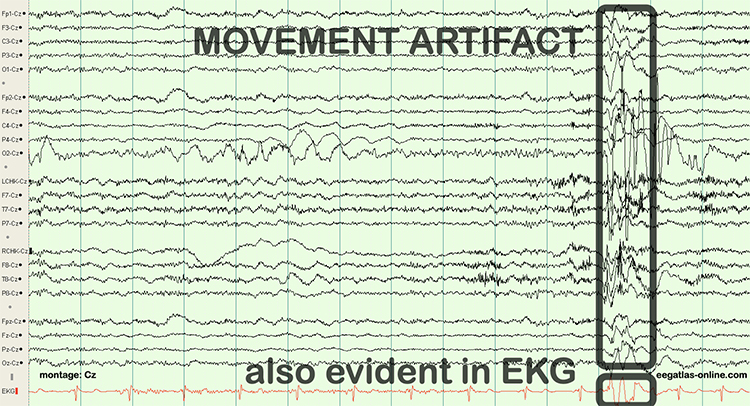
The graphic below shows movement artifacts due to head movement (left), respiration (center), and tongue movement (right).
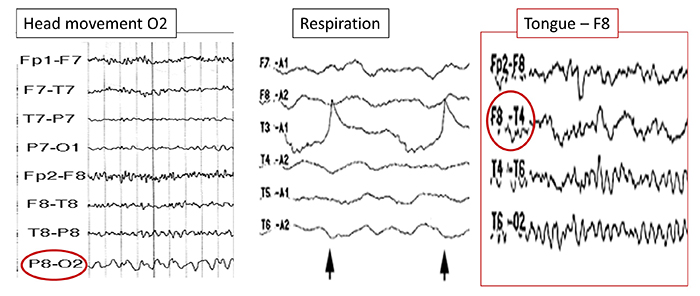
Below is a BioGraph ® Infiniti cable movement artifact display. Note the two voltage spikes at the beginning of the recording.
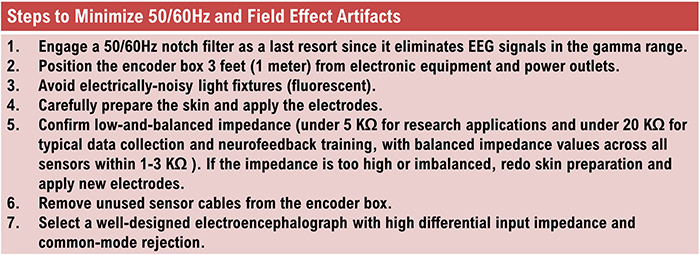
50/60 Hz and Field Artifacts
Both 50/60 Hz and field artifacts are external artifacts transmitted by nearby electrical sources. While 60-Hz artifact is a risk in North America where AC voltage is transmitted at 60 Hz, 50-Hz artifact is a problem in other locations that generate power at 50 Hz. Their fundamental frequency is 50 or 60 Hz with harmonics at 100/120 Hz, 150/180 Hz, and 200/240 Hz. Imbalanced electrode impedances increase an EEG amplifier's vulnerability to these artifacts. The 60-Hz artifact graphic below © John S. Anderson.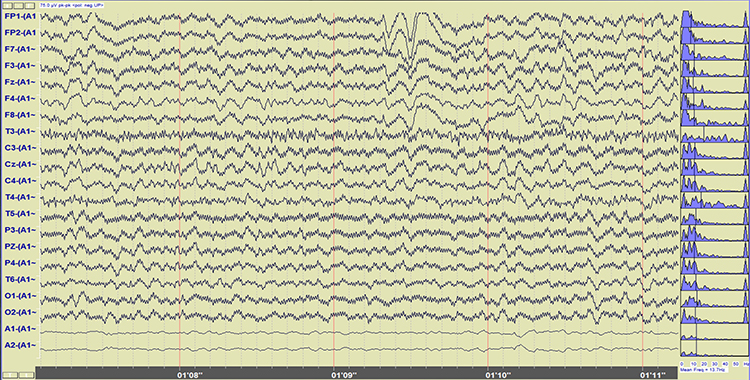
A BioGraph ® Infiniti display of 60-Hz artifact is shown below in red. Note the cyclical voltage fluctuations and 60-Hz peak in the power spectral display.
Radiofrequency Artifact
Radiofrequency (RF) artifact radiates outward like a cone from the front of televisions and computer monitors. Graphic © eegatlas-online.com.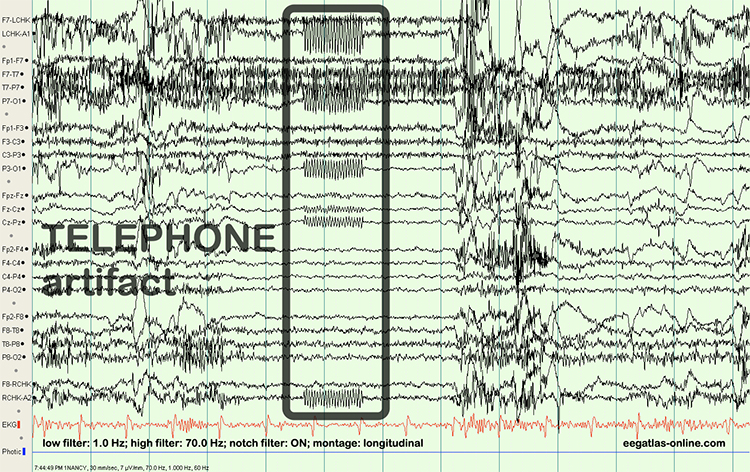
 Artifacts.jpg)
Electrode Artifacts
There are several sources of electrode artifacts. Even with proper care, electrode surfaces can become corroded and the leads and connectors damaged. Using sensors with mismatched electrode metals can cause polarization of amplifier input stages.Impedance Artifact
Unless skin-electrode impedance is low (under 5 KΩ for research and 20 KΩ for training) and balanced (under 1-3 KΩ ), diverse artifacts like 50/60 Hz and movement can contaminate the EEG signal, as seen in the P3 and Pz electrodes. Graphic © eegatlas-online.com.
Electrode Pop Artifact
Even when the impedance is low and balanced, mechanical disturbance can produce a unique artifact. Electrode pop artifact has a sudden large deflection in at least one channel when an electrode abruptly detaches from the scalp. This may also happen when there is a bubble or other defect in the gel or paste, and a charge builds up and subsequently "jumps" across the gap, resulting in a large electrical discharge. Graphic © John S. Anderson.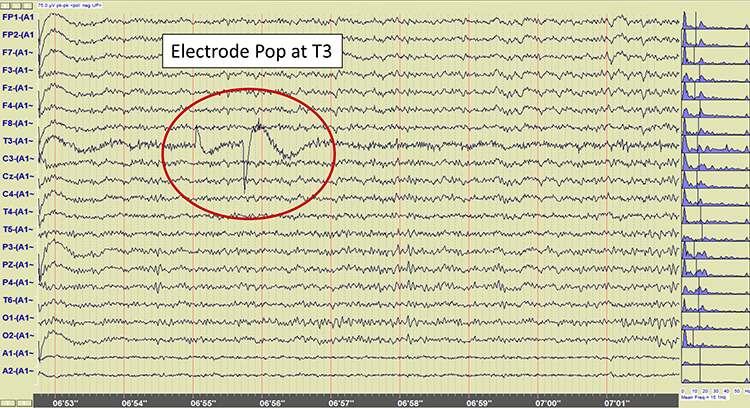
Glossary
50/60 Hz: external artifacts transmitted by nearby electrical sources.
A (auricular): International 10-20 system earlobe reference placement.
abnormal periodic paroxysmal patterns: generalized periodic paroxysmal patterns and lateralized periodic paroxysmal patterns.
abnormal slow activity: generalized intermittent slow activity, focal and lateralized intermittent slow activity, and persistent slow activity.
active electrode: an electrode placed over a site that is a known EEG generator like Cz.
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
artifact: false signals like 50/60Hz noise produced by line current.
benign epileptiform transients of sleep (BETS): sharp waves are seen alone or as a low-amplitude spike and a smaller after-going slow wave. BETS can be monophasic or diphasic and occur during light sleep. There is no disturbance of background activity, and it does not progress.
benign small sharp spikes (BSSS): sharp waves are seen alone or as a low-amplitude spike and a smaller after-going slow wave. BETS can be monophasic or diphasic and occur during light sleep. There is no disturbance of background activity, and it does not progress.
bilateral independent periodic discharges (BIPDs): asynchronous discharges that occur independently in the left and right hemispheres, appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz.
bilateral independent periodic lateralized epileptiform discharges (BIPLEDs): asynchronous discharges that occur independently in both hemispheres, appear as sharp waves or spikes, 40-100 µV in bipolar montages, and recur at rates from 0.5-1.5 Hz.
bridging artifact: a short circuit between adjacent electrodes due to excessive application of electrode paste or a client who is sweating excessively or who arrives with a wet scalp.
brief duration: 10-60 s.
C (central): sites in the International 10-20 system that detect frontal, parietal-occipital activity and temporal EEG activity.
cardiac artifact: the contamination of the EEG by the ECG signal.
channel: an EEG amplifier input from three leads (active, reference, and ground electrodes) placed on the head.
drowsiness artifact: in adults, 1-Hz (or slower) waveforms can be detected with greatest amplitude and reverse polarity at F7 and F8 may progress to 1-2 Hz slowing of the alpha rhythm.
EEG artifacts: noncerebral electrical activity in an EEG recording can be divided into physiological and exogenous artifacts.
electro-ocular artifact: contamination of EEG recordings by potentials generated by eye blinks, eye flutter, and eye movements.
electrode: a specialized conductor that converts biological signals like the EEG into currents of electrons.
electrode pop artifact: sudden large deflections in at least one channel when an electrode abruptly detaches from the scalp.
EMG artifact: interference in EEG recording by volume-conducted signals from skeletal muscles.
epileptogenic patterns of doubtful significance: brief EEG patterns not associated with seizures or neurological disorders. Examples are 6-Hz spike-and-slow-wave, 14- and 16-Hz positive bursts, benign epileptiform transients of sleep (BETS), rhythmical mid-temporal discharge (RMTD), small sharp spikes (SSS), and wicket spikes.
evoked potential artifact (event-related potential artifact): somatosensory, auditory, and visual signal processing-related transients that may contaminate multiple channels of an EEG record.
exogenous artifacts: noncerebral electrical activity generated by movement, 50/60 Hz and field effect, bridging, and electrode (electrode “pop" and impedance) artifacts.
field artifacts: external artifacts transmitted by nearby electrical sources.
focal and lateralized intermittent slow activity: EEG activity under 8 Hz and usually confined to a single or a couple of adjacent electrodes. These bursts have an irregular appearance, are composed of several frequencies, and rarely involve an entire hemisphere.
F (frontal): sites in the International 10-20 system that detect frontal lobe EEG activity.
Fp (frontopolar or prefrontal): sites in the International 10-20 system that detect prefrontal cortical EEG activity.
frontal intermittent rhythmic delta activity (FIRDA): bilateral and synchronous discharges that exceed 100 µV and recur at rates up to 3 Hz.
frequency (Hz): the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
generalized intermittent slow activity: asynchronous EEG activity under 8 Hz that involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta.
generalized periodic discharges (GPDs): asynchronous EEG activity under 8 Hz that involves the majority or all of both hemispheres. These bursts typically consist of polymorphic delta.
generalized periodic epileptiform discharges (GPEDs or GPDs): bilateral and synchronous discharges that exceed 100 µV and recur at rates up to 3 Hz.
generalized periodic paroxysmal patterns: epileptiform discharges in the same areas of both hemispheres. The waveforms exhibit similar composition, amplitude, and phase in each hemisphere but may slightly vary within a hemisphere.
generalized rhythmic delta activity (GRDA): generalized rhythmic delta activity. The term frontal intermittent rhythmic delta activity (FIRDA) was used before ACNS standardization in 2012. These bilateral and synchronous discharges exceed 100 µV and recur at rates up to 3 Hz.
hertz (Hz): the unit of frequency measured in cycles per second.
high amplitude: 60-200+ µV.
ictal epileptiform discharges: individual spikes and sharp waves, and complexes that contain both waveforms that last less than 2 s.
impedance (Z): the complex opposition to an AC signal measured in Kohms.
impedance meter: a device that uses an AC signal to measure impedance in an electric circuit, such as between active and reference electrodes.
impedance test: automated or manual measurement of skin-electrode impedance.
inion: bony prominence on the back of the skull.
interictal epileptiform discharges: high-amplitude spike-and-wave or polyspike-and-wave complexes that repeat at a frequency of 3 Hz.
intermediate duration: 1-1.5 minutes.
intermittent rhythmic delta activity (IRDA): bilateral synchronous discharges recur between 2-2.5 Hz and appear in brief bursts.
International 10-20 system: a standardized procedure for 21 recording and one ground electrode on adults.
lateralized periodic discharges (LPDs): lateralized periodic discharges. These unilateral discharges appear as sharp waves or spikes, 100-300 µV, and recur at rates up to 3 Hz.
lateralized periodic paroxysmal patterns: epileptiform discharges that differ from generalized periodic paroxysmal patterns in their unilateral distribution. Both patterns share the same waveform morphology.
lateralized rhythmic delta activity (LRDA): unilateral discharges recur at rates up to 3 Hz. LRDA runs typically last less than 1 minute and is shorter than LPDs.
low amplitude: less than 20 µV.
mastoid bone: the bony prominence behind the ear.
microvolt (μV): the unit of amplitude (signal strength) that is one-millionth of a volt.
movement artifact: voltages caused by client movement or the movement of electrode wires by other individuals.
multifocal (Mf): an EEG abnormality detected at several scalp locations.
nasion: the depression at the bridge of the nose.
notch filter: a filter that suppresses a narrow band of frequencies, such as those produce by line current at 50/60Hz.
O (occipital): sites in the International 10-20 system that detect occipital lobe EEG activity.
ohm (Ω): unit of impedance or resistance.
P (parietal): sites in the International 10-20 system that detect parietal lobe EEG activity.
paroxysmal epileptogenic abnormalities: interictal epileptiform discharges (focal, generalized), ictal, secondary bilateral synchrony, and epileptiform patterns of doubtful significance.
periodic lateralized epileptiform discharges (PLEDs): lateralized or focal and exhibit regular periodic, negative spike-and-sharp wave patterns with a 20-1000 ms duration and 50-300 µV amplitude.
physiological artifacts: noncerebral electrical activity that includes electromyographic, electro-ocular (eye blink and eye movement), cardiac (pulse), sweat (skin impedance), drowsiness, and evoked potential.
polarization: chemical reactions produce separate regions of positive and negative charge where an electrode and electrolyte make contact, reducing ion exchange.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
preauricular point: the slight depression located in front of the ear and above the earlobe.
prolonged duration: 5-60 minutes.
protracted duration: greater than 60 minutes.
pulse artifacts: noncerebral voltages due to mechanical movement of an electrode in relation to the skin surface due to the pressure wave of each heartbeat.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
rhythmic temporal theta of drowsiness (RMTD): bitemporal left is greater than right in this longitudinal bipolar montage. Noted are notched rhythmic waveforms localized to the temporal regions, some of which are sharply contoured.
secondary bilateral synchrony (SBS): spikes with a single phase reversal around the midline.
sharp-wave (SW): transient with pointed peak and 70 to 200 ms duration.
spike-wave (SW): transient with pointed peak and 20 to under 70 ms duration.
SREDA (or SCREDA): sub-clinical rhythmic electrographic discharges in adults.
stimulus induced rhythmic, periodic, or ictal discharges (SIRPIDs): EEG discharges reliably produced by alerting stimuli.
sweat artifact: changes in the EEG signal when sweat on the skin changes the conductive properties under and near the electrode sites (i.e., bridging artifact).
tragus: the flap at the opening of the ear.
transient: isolated waveforms or complexes that can be distinguished from background activity.
typical amplitude: 20-50 µV, depending on age.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
very brief duration: less than 10 s.
wicket waves: EEG activity with arciform appearance, no after-going slow-wave, and no background disruption or disturbance.
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

REVIEW FLASHCARDS ON QUIZLET PLUS
Click on the Quizlet Plus logo to review our chapter flashcards.

Visit the BioSource Software Website
BioSource Software offers Functional Neuroanatomy, which reviews critical behavioral networks, and qEEG100, which provides extensive multiple-choice testing over the IQCB Blueprint.


Assignment
Now that you have completed this module, summarize your key talking points when explaining the process of NFT to a client. Which concepts do your clients find most challenging? Which explanations have been most effective?
References
Benbadis, S. R., & Rielo, D. A. (2018). Encephalopathic EEG patterns. Medscape. Retrieved from https://emedicine.medscape.com/article/1140530-overview.
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer (3rd ed.). Elsevier.
Ferree, T. C., Luu, P., Russell, G. S., & Tucker, D. M. (2001). Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology, 112(3), 536–544. https://doi.org/10.1016/s1388-2457(00)00533-2
ISNR Board of Directors (2010). What is neurofeedback? Retrieved from https://isnr.org/what-is-neurofeedback.
Jin, L. (2007). A reappraisal of secondary bilateral synchrony. Neurology Asia, 12, 29-35.
Johnson, E. L., & Kaplan, P. W. (2017). Population of the ictal-interictal zone: The significance of periodic and rhythmic activity. Clin Neurophysiol Pract, 2, 107-118. https://doi.org/10.1016/j.cnp.2017.05.001
Kappenman, E. S., & Luck, S. J. (2010). The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology, 47(5), 888-904. https://doi.org/10.1111/j.1469-8986.2010.01009.x.
Khazan, I. Z. (2019). Biofeedback and mindfulness in everyday life: Practical solutions for improving your health and performance. W. W. Norton & Company.
Neurofeedback essential skills list. (2020). Biofeedback Certification International Alliance.
Peper, E., Gibney, K. H., Tylova, H., Harvey, R., & Combatalade, D. (2008). Biofeedback mastery: An experiential teaching and self-training manual. Association for Applied Psychophysiology and Biofeedback.
Richardson, C. A., & Benbadis, S. R. (2019). Generalized EEG waveform abnormalities. Medscape. Retrieved from https://emedicine.medscape.com/article/1140075-overview.
G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
